Superior clinical and economic performance
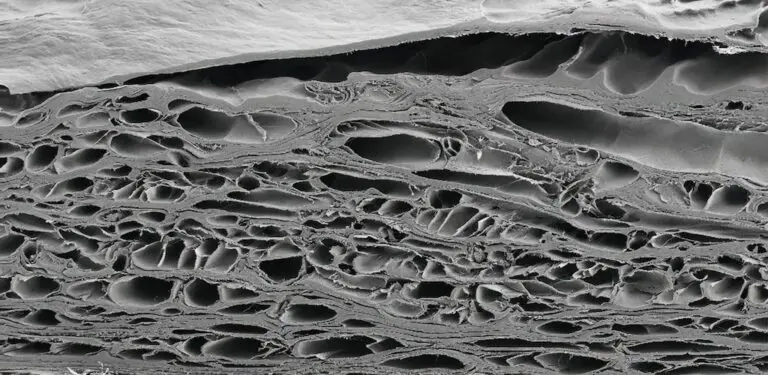
Thousands of healthcare providers across the globe trust Kerecis fish-skin technology to treat their patients. Our minimally processed fish-skin grafts are homologous to human skin both in terms of structure and molecular content, with ample supply of collagen, elastin, proteoglycans, glycans, and lipids.[1-3] When applied to damaged tissue, such as burns or wounds, Kerecis grafts support the body’s own cells to regenerate and repair tissue.[4-6]
We continuously adapt to our customers’ needs and expectations by offering a diverse range of product variations that deliver financial advantages. Kerecis fish-skin products are FDA-cleared for multiple clinical applications, including partial and full-thickness wounds, trauma wounds, and surgical wounds. Our grafts can be expanded to cover larger wounds with less fish-skin material, creating cost efficiencies and reducing waste.