MariGen is intact fish skin which is used to support tissue regeneration in surgical, traumatic, and chronic wounds.3 Because there is no known risk of viral or prion disease transfer from North Atlantic cod to humans, the fish skin only needs mild processing with our proprietary method.2 This process preserves the skin’s natural qualities2, including: its three-dimensional structure, mechanical properties, molecular organization, and composition.1-4 MariGen promotes healing with minimal impairment of functionality and positive cosmetic outcomes.4 The product is homologous to human skin1 and when applied to damaged tissue, such as a chronic wounds, provides the perfect environment to support the body’s own cells to regenerate tissue.5-12
Intact fish skin which is used to support tissue regeneration in surgical, traumatic, and chronic wounds.3
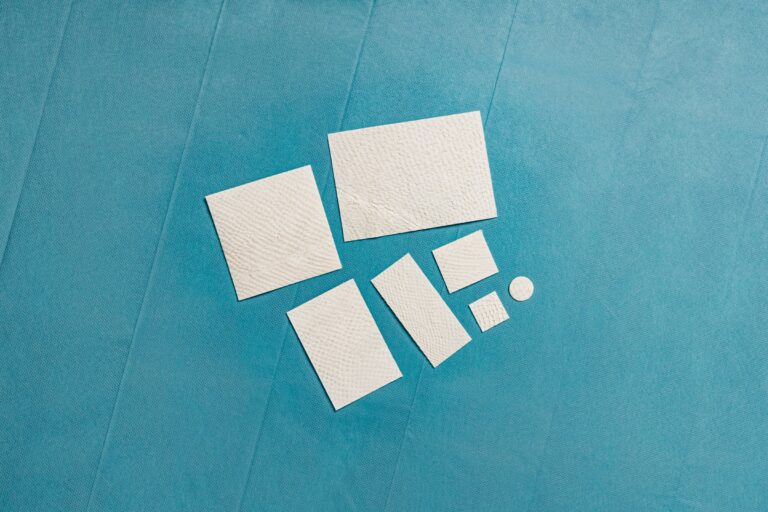
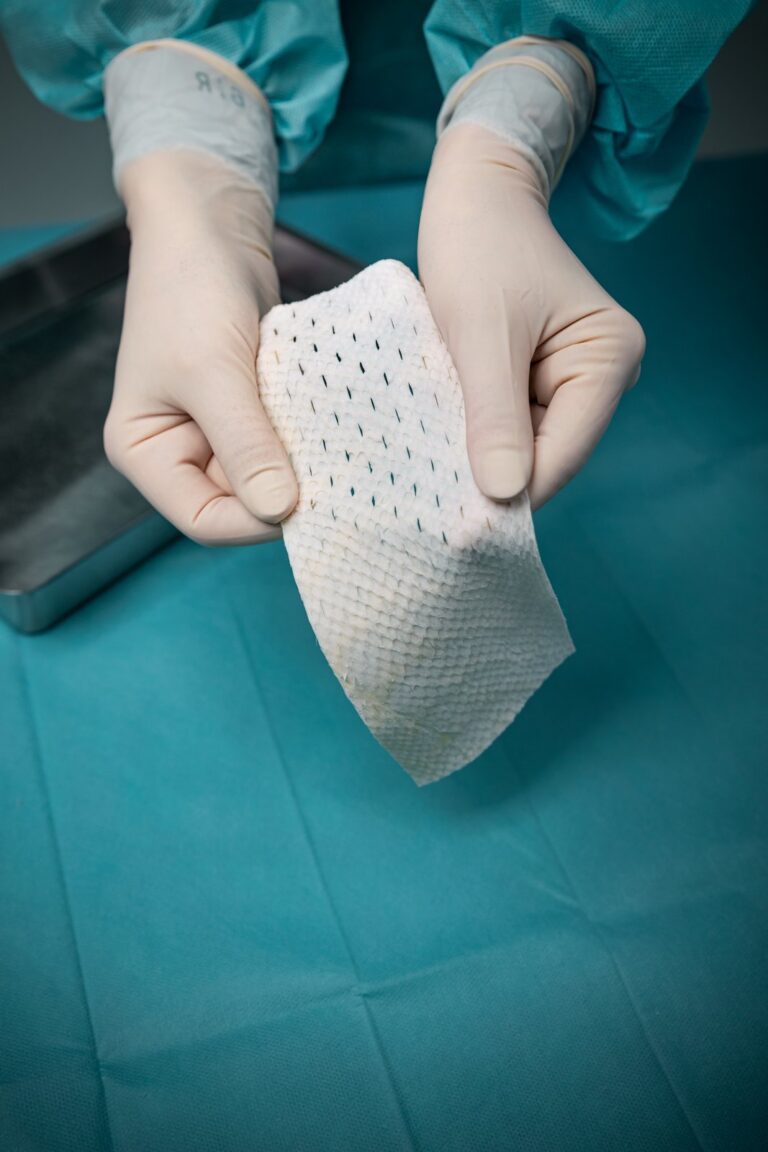
Intact fish skin that has been fragmented. It offers more surface area than the non-fragmented version, and is designed to adhere to, and fill, deep wound spaces and irregular geometries.

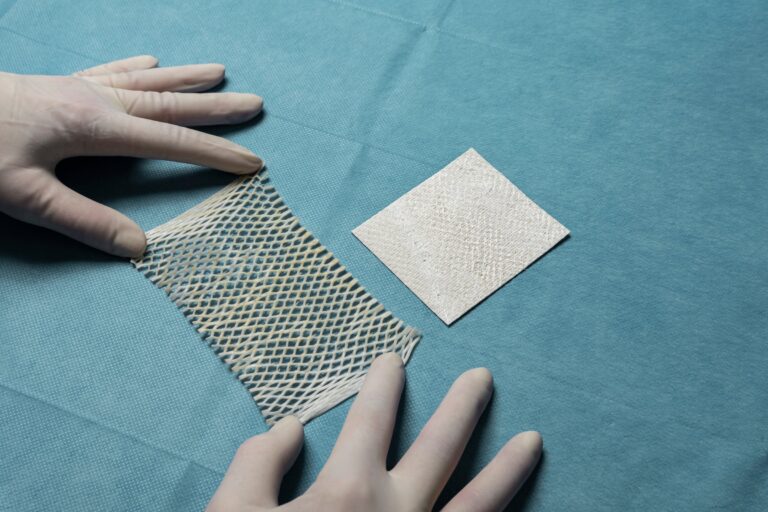
The pre-fenestrated version of MariGen facilitates easy and effective drainage of excess exudate, while still allowing the fish skin to maintain total contact with the wound bed.
2:1 pre-meshed intact fish skin graft for supporting healing and tissue regeneration of chronic wounds. MariGen Expanse is specially designed to expand to cover wounds of 100 cm2 and larger that are treated in an out-patient care setting.
Magnusson, S. et al. Regenerative and Antibacterial Properties of Acellular Fish Skin Grafts and Human Amnion/Chorion Membrane: Implications for Tissue Preservation in Combat Casualty Care. Mil. Med. 182, 383–388 (2017).
Baldursson, B. T. et al. Healing rate and autoimmune safety of full-thickness wounds treated with fish skin acellular dermal matrix versus porcine small-intestine submucosa: a non-inferiority study. Int. J. Low. Extrem. Wounds 14, (2015).
Magnusson, S. et al. Decellularized fish skin: characteristics that support tissue repair. Laeknabladid 101, 567–573 (2015).
Alam, K. & Jeffery, S. L. A. Acellular Fish Skin Grafts for Management of Split Thickness Donor Sites and Partial Thickness Burns: A Case Series. Mil Med. 184(Suppl 1):16-20 (2019).
Pujji, O. & Jeffery, S. L. A. Safe burn excision prior to military repatriation: an achievable goal? BMJ Military Health.164: 358-359 (2018).
Shupp, J. W. et al. Fish Skin Compared to Cadaver Skin as a Temporary Covering for Full Thickness Burns: An Early Feasibility Trial. Poster presented at: AMUS 2020 Annual Meeting (2020).
Badois, N. et al., Acellular fish skin matrix on thin-skin graft donor sites: a preliminary study. J Wound Care 28. 624–628 (2019).
Kirsner, R. S. et al. Fish skin grafts compared to human amnion/ chorion membrane allografts: A double-blind, prospective, randomized clinical trial of acute wound healing. Wound Repair Regen. 28, 75–80 (2020).
Lantis, J.C, 2nd, et al. Final efficacy and cost analysis of a fish skin graft vs standard of care in the management of chronic diabetic foot ulcers: a prospective, multicenter, randomized controlled clinical trial. Wounds. 35(4): 71-79 (2023).
Wallner, C. et al. A Comparison of Intact Piscine Skin, Split-thickness Skin Graft, and Lactic Acid Membrane in Treating Superficial and Deep Burn Wounds Following Enzymatic Debridement, J Burn Care Res. 42 (Suppl 1): 125-126 (2021).
Stone, R. 2nd, et al. Omega-3 Rich Fish Skin Grafts Reduce Donor Skin Requirements for Full Thickness Burns. J. Burn Care Res. 39, S234–S235 (2018).
Stone, R. 2nd, et al. Accelerated Wound Closure of Deep Partial Thickness Burns with Acellular Fish Skin Graft. Int J Mol Sci. 2021;22(4):1590 (2021).
Evangelatov, A., & Pankov, R. The evolution of three-dimensional cell cultures towards unimpeded regenerative medicine and tissue engineering. In Andrades, J. A. [Ed.] Regenerative medicine and tissue engineering. IntechOpen (2013).
Wang, Y. et al. Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Advanced drug delivery reviews, 123, 3-17 (2018).