Shield® is intact fish skin indicated for the management of chronic and acute wounds such as diabetic foot ulcers, pressure ulcers, vascular ulcers, post-Moh’s surgical wounds, and draining wounds commonly treated in physician offices.
Shield is comprised of an intact fish skin graft and an integral non-adherent silicone contact layer. The graft is fenestrated, and the contact layer is porous, to allow for proper exudate management.
The contact layer is designed to be hydrated with the graft, and shields the graft from getting caught within secondary dressings, and from exudate getting caught between the graft and dressings. It also provides added workflow efficiency by preventing the need to source an optimum contact layer and contour it to size and shape.
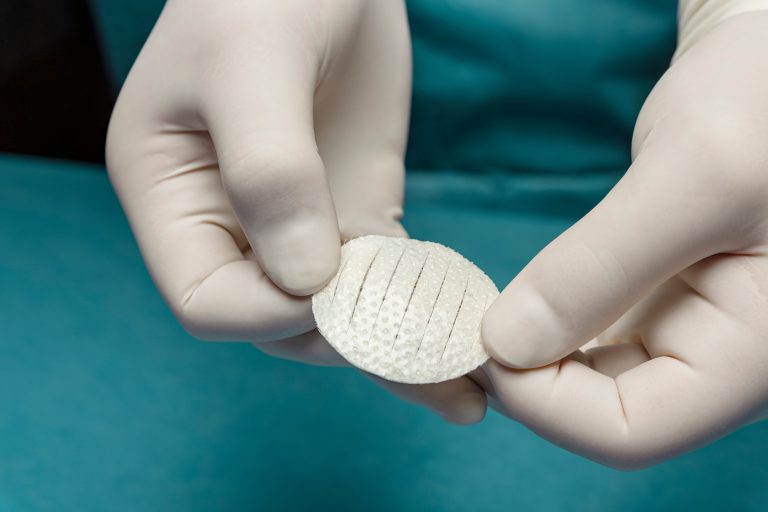
Shield Standard features a borderless silicone contact layer protecting the intact fish skin. It allows for cutting the fish skin and protective silicone to easily fit inside the wound edges and prevents the product from tenting over deeper wounds.
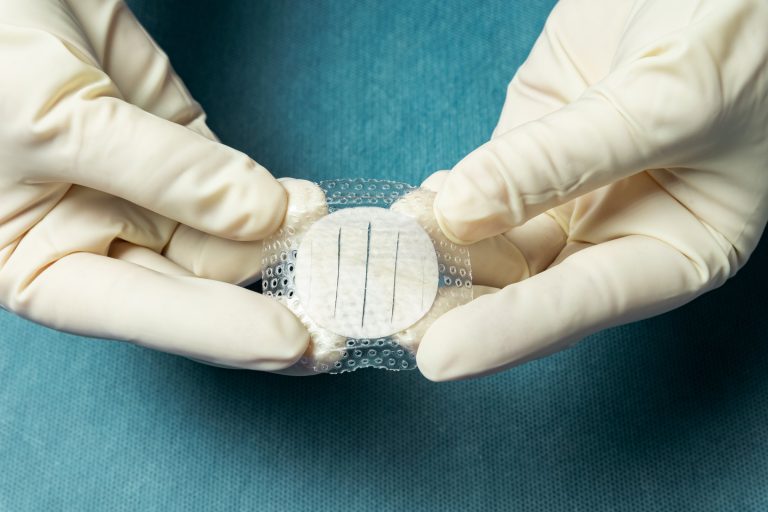
Shield Adhesive is intact fish skin featuring an integrated silicone contact layer and border. It provides the optimal gentle adhesive while allowing for moisture control and protection of the fish skin graft.
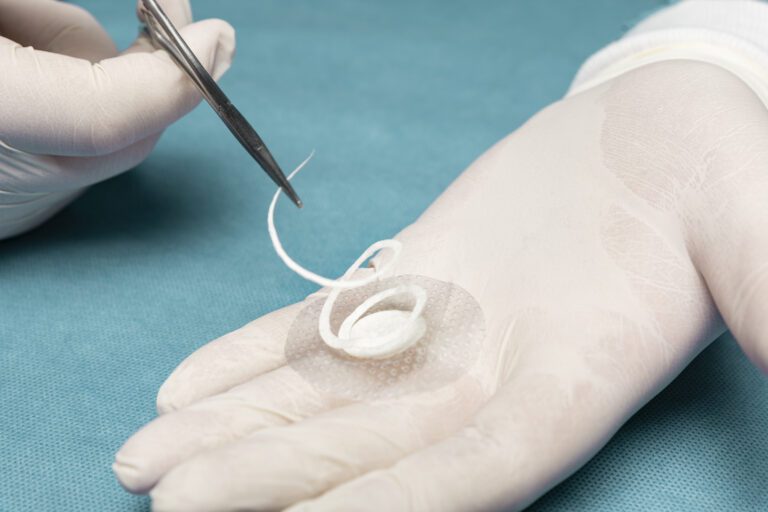
Shield Spiral features perforated fenestration marks cut in a spiral pattern specially designed for easy & flexible graft tailoring to accommodate a range of wound sizes and shapes.