
From the Pure Waters of Iceland
Intact Fish Skin Grafts for Tissue Regeneration
SurgiBind®
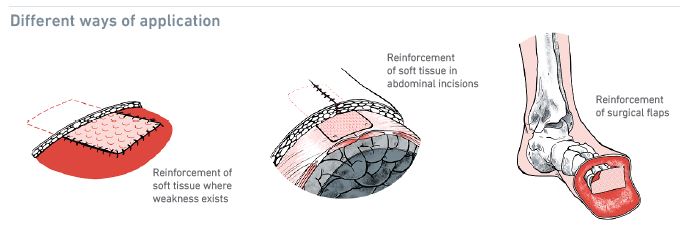
Kerecis SurgiBind is intact fish-skin mesh intended for surgical implantation in plastic and reconstructive surgery. The product can be used to reinforce soft tissue where weakness exists.
The Kerecis fish-skin mesh contains fat, protein, elastin, glycans and other natural tissue elements and are supplied in multiple shapes, variants and sizes.
The product classifies as a medical device and consist of a full thickness fish-skin that has been processed using Kerecis’ proprietary EnviroIntact™ method. The product is not subject to the May 31, 2021, FDA 351 regulation.
Indications
- For implantation to reinforce soft tissue where weakness exists, in patients requiring soft tissue repair, or reinforcement in plastic or reconstructive surgery.
The intact fish-skin mesh in various sizes
| wdt_ID | wdt_created_by | wdt_created_at | wdt_last_edited_by | wdt_last_edited_at | SKU | Product Description | Product Sizing | Total Units |
|---|---|---|---|---|---|---|---|---|
| 1 | avista | 27/02/2024 22:00 | avista | 28/02/2024 15:45 | 50241S02D0D | SurgiBind 3×7 cm | 3 x 7 cm | 21 |
| 2 | avista | 27/02/2024 23:33 | avista | 28/02/2024 15:45 | 50241S10D0D | SurgiBind 3×12 cm | 3 x 12 cm | 36 |
| 3 | avista | 28/02/2024 15:45 | avista | 28/02/2024 15:45 | 50241S03D0D | SurgiBind 7×10 cm | 7 x 10 cm | 70 |
| 4 | avista | 28/02/2024 15:46 | avista | 28/02/2024 15:46 | 50241S21D0D | SurgiBind 7×20 cm | 7 x 20 cm | 140 |
Intact fish-skin graft fenestrated to allow for easier flow through of liquids.
| wdt_ID | wdt_created_by | wdt_created_at | wdt_last_edited_by | wdt_last_edited_at | SKU | Product Description | Product Sizing | Total Units |
|---|---|---|---|---|---|---|---|---|
| 1 | avista | 27/02/2024 22:00 | avista | 28/02/2024 15:49 | 50241G02D0D | SurgiBind Fenestrated 1:1 3×7 cm | 3 X 7 cm fenestrated 1:1 | 21 |
| 2 | avista | 27/02/2024 23:33 | avista | 28/02/2024 15:50 | 50241G10D0D | SurgiBind Fenestrated 1:1 3×12 cm | 3 X 12 cm fenestrated 1:1 | 36 |
| 3 | avista | 28/02/2024 15:45 | avista | 28/02/2024 15:50 | 50241G03D0D | SurgiBind Fenestrated 1:1 7×10 cm | 7 x 10 cm fenestrated 1:1 | 70 |
| 4 | avista | 28/02/2024 15:46 | avista | 28/02/2024 15:50 | 50241G21D0D | SurgiBind Fenestrated 1:1 7×20 cm | 7 x 20 cm fenestrated 1:1 | 140 |
| 5 | avista | 28/02/2024 15:46 | avista | 28/02/2024 15:50 | 50241G24D0D | SurgiBind Fenestrated 1:1 250 sq cm | 250 sq cm fenestrated 1:1 | 250 |
Contraindications
The product derives from a from a fish source and should not be used in patients with a known allergy to fish or sensitivity. Note that fish-allergy is not the same as shellfish-allergy.
The product is not indicated for repairs where load bearing support from the mesh is required such as in the repair of any hernia. The device is not indicated for intraperitoneal organ contact. The device is not indicated for bridging effects.
Availability & Indications
The product and its variations are not available in all countries and may be known under different trade names. Products, product names, indications and claims vary between jurisdictions. Contact your Kerecis representative if you have questions about the availability of Kerecis products in your area.
Always make sure to check with your local health care professional and read the actual printed package information prior to use. Information on this website might not be current and might not be an accurate reflection of current product labeling.
Not Healthcare Advice
This website is not intended to provide diagnosis, treatment or medical advice. Products, services, information and other content provided on this website, including information that may be provided on directly or by linking to third-party websites, are provided for informational purposes only. Information on this website should not be considered as a substitute for advice from a healthcare professional.
Consult with a physician or other healthcare professional regarding any medical or health related diagnosis or treatment options.
Surgeon Disclaimer
A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Kerecis does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before using it in surgery.
The information presented on this website are intended to demonstrate the Kerecis product offerings. A surgeon must always refer to the package insert, product label and/or instructions for use before using any Kerecis product.


