The Only Intact Fish Skin Graft: Managing Challenging Chronic and Acute Wounds.
Backed by robust clinical data including the landmark Odinn Study
Included in CMS LCDs for skin substitutes in the treatment of diabetic foot ulcers (DFUs)
Supported by a validated IFU allowing use over bone and tendon
FDA-cleared and available through both Medicare and commercial insurers like Cigna, Anthem, BCBS

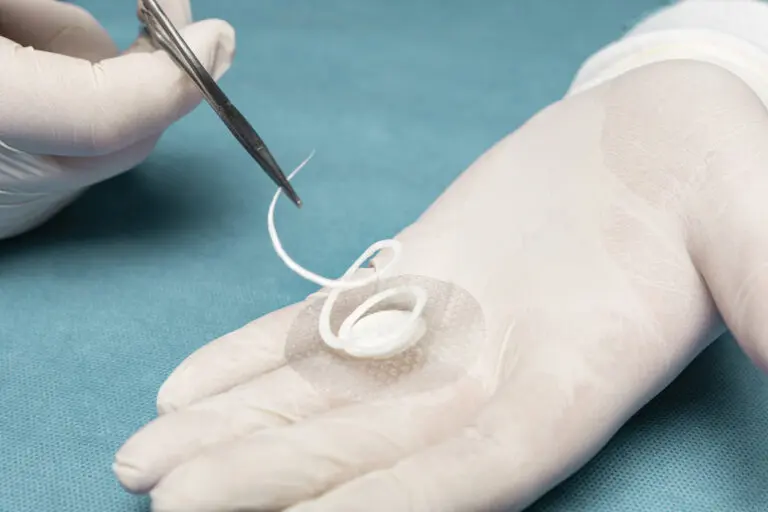
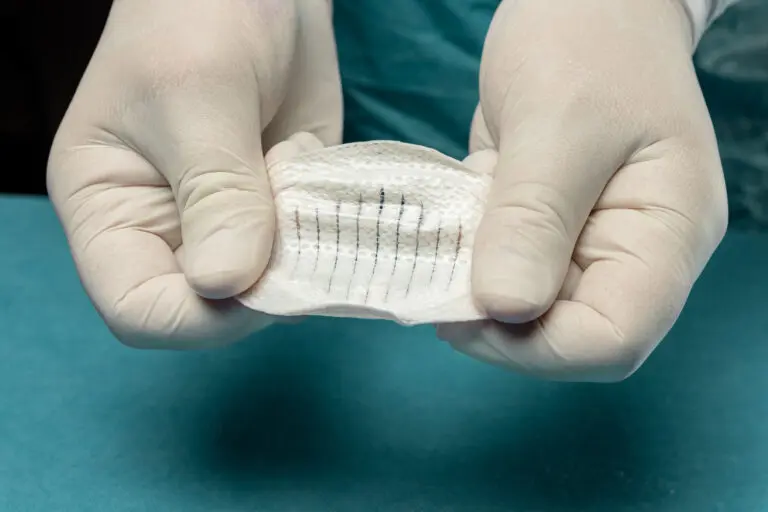
Kerecis Shield® Products
Shield® is intact fish skin indicated for the management of chronic and acute wounds such as diabetic foot ulcer wounds, pressure ulcers, vascular ulcers, post-Moh’s surgical wounds, and draining wounds commonly treated in physician’s private offices.
The grafts contain natural elements, and are fenestrated to allow adequate wound drainage for appropriate wound moisture at the wound bed. Shield products are integrated with a silicone contact layer to protect the wound and for added workflow efficiency.
Shield products are classified as medical devices, and consist of intact fish skin that has been processed using Kerecis’ proprietary EnviroIntact™ method.
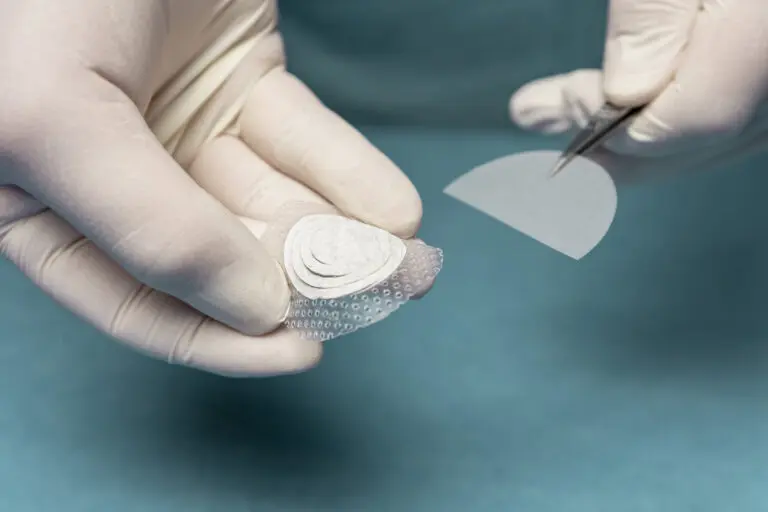
Shield® Spiral
Shield Spiral features perforated fenestration marks cut in a spiral pattern specially designed for easy & flexible graft tailoring to accommodate a range of wound sizes and shapes.
Shield® Adhesive
Shield Adhesive is intact fish skin featuring an integrated silicone contact layer and border. It provides the optimal gentle adhesive while allowing for moisture control and protection of the fish skin graft.

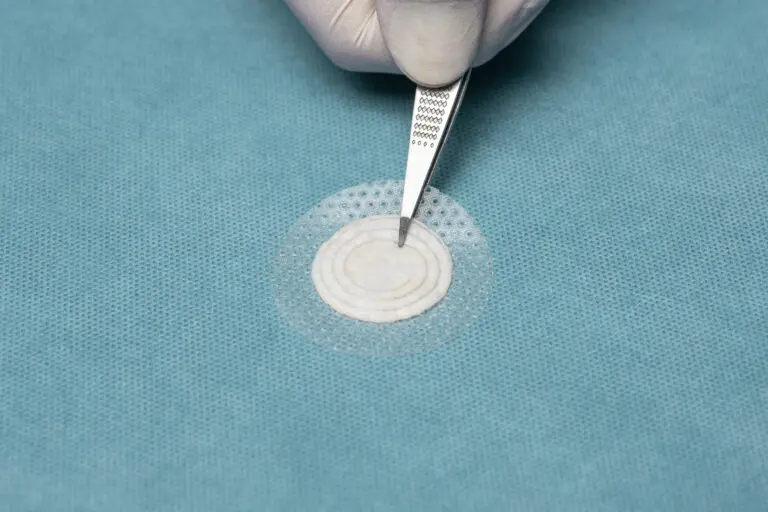
Shield® Standard
Shield Standard features a borderless silicone contact layer protecting the 50 mm intact fish skin. It allows for cutting the fish skin and protective silicone to easily fit inside the wound edges and prevents the product from tenting over deeper wounds
The Science Behind the Skin
Kerecis grafts have been the subject of 90+ peer-reviewed studies — including multiple randomized controlled trials — showing faster healing rates and superior clinical outcomes for chronic wounds.
Highlighted Studies
Extensive Payer Coverage. Real-Time Eligibility Support.
Kerecis is currently covered by Anthem BCBS, Humana and now Cigna for the treatment of DFUs. With our Kerecis Current™ tool, clinicians can instantly confirm patient eligibility through their EMR systems — no guessing, no delays.
Key Reimbursement Highlights:
- CMS LCDs remain active through January 2026
- Kerecis MariGen® and Shield® are FDA-cleared for multiple wound types
- Eligible for use in Part B and commercial plans nationwide
- Use is considered reasonable and necessary under most LCDs and clinical documentation standards
Benefits of using Kerecis Shield®
Known Challenges in Wound Care
Optimum application of biologic solutions is dependent on the right environment, including:
Timely Graft Removal. Biologics are highly active in the wound bed, so providers need to prevent the graft from getting stuck in a covering dressing, or being accidentally removed by someone on the care team.
Similarly, providers need to avoid the risk of exudate getting stuck under the graft or dressing: Diabetic Foot Ulcers (DFUs) and other lower extremity wounds may have increased exudate initially when biologic grafts are used, so ensuring the exudate doesn’t get trapped under the graft or dressing is essential for best outcomes.
Time is everything for healthcare providers. Gathering the ideal contact layer or covering dressing is not just time consuming, it can be infeasible in certain situations.
Shield use in Wound Management
The Solution: Shield is specially designed to help address these challenges.
A protective “shield” directly on the fish skin graft helps ensure the fish skin does not get stuck in covering dressings, and allow for optimal drapability so it conforms to the wound. The contact layer on Shield Adhesive helps keep the graft in place for the initial placement, then separate from the graft and allow for removal of the silicone without pulling away the graft.
Exudate management is the cornerstone of all good wound care. A silicone without proper exudate control could trap fluids and risk pooling of exudate, infections or hematomas forming. To help reduce this risk, Shield products have specially-designed fenestration lines in the fish skin graft, and extra large pores in the silicone. This allows for maximum fish skin contact with the wound, and maximum drainage while still providing protective coverage.
All included in a single product, Shield helps to protect the wound environment while speeding up the preparation and application process, helping to ensure patients get the best possible outcome from their fish skin graft application.
Indications Include
- Diabetic Ulcers
- Venous Ulcers
- Pressure Ulcers
- Surgical Wounds (including post-Mohs, donor sites/grafts)
- Full-thickness wounds (extending through the dermis to deeper tissues such as subcutaneous fat, muscle, tendon or bone)
- Partial-thickness wounds (extending through the epidermis or into dermis)
Other Kerecis Products
Leader in Clinical Studies
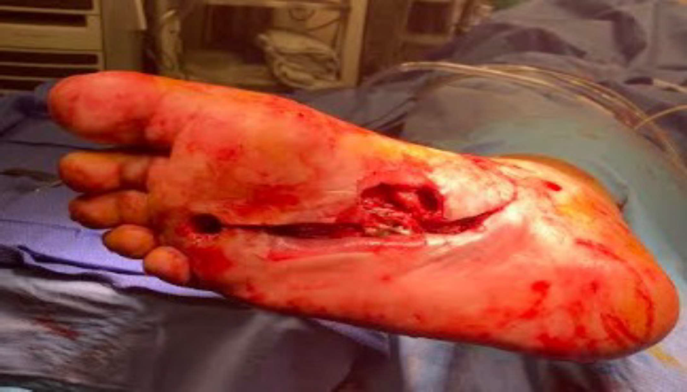
Diabetic Foot Wound
Patient History, Initial Treatment
- 58-year-old male
- Past medical history of type II diabetes, previously treated for two months for right foot sub 4th metatarsal head ulcer
- The patient presented to the emergency room febrile and septic, the wound was draining and positive for ecchymosis, edema, and erythema
- Patient was treated with repeat surgical incisional and drainage, 4th metatarsal head resection, and application of antibiotic beads
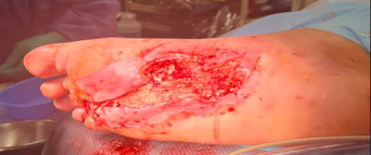
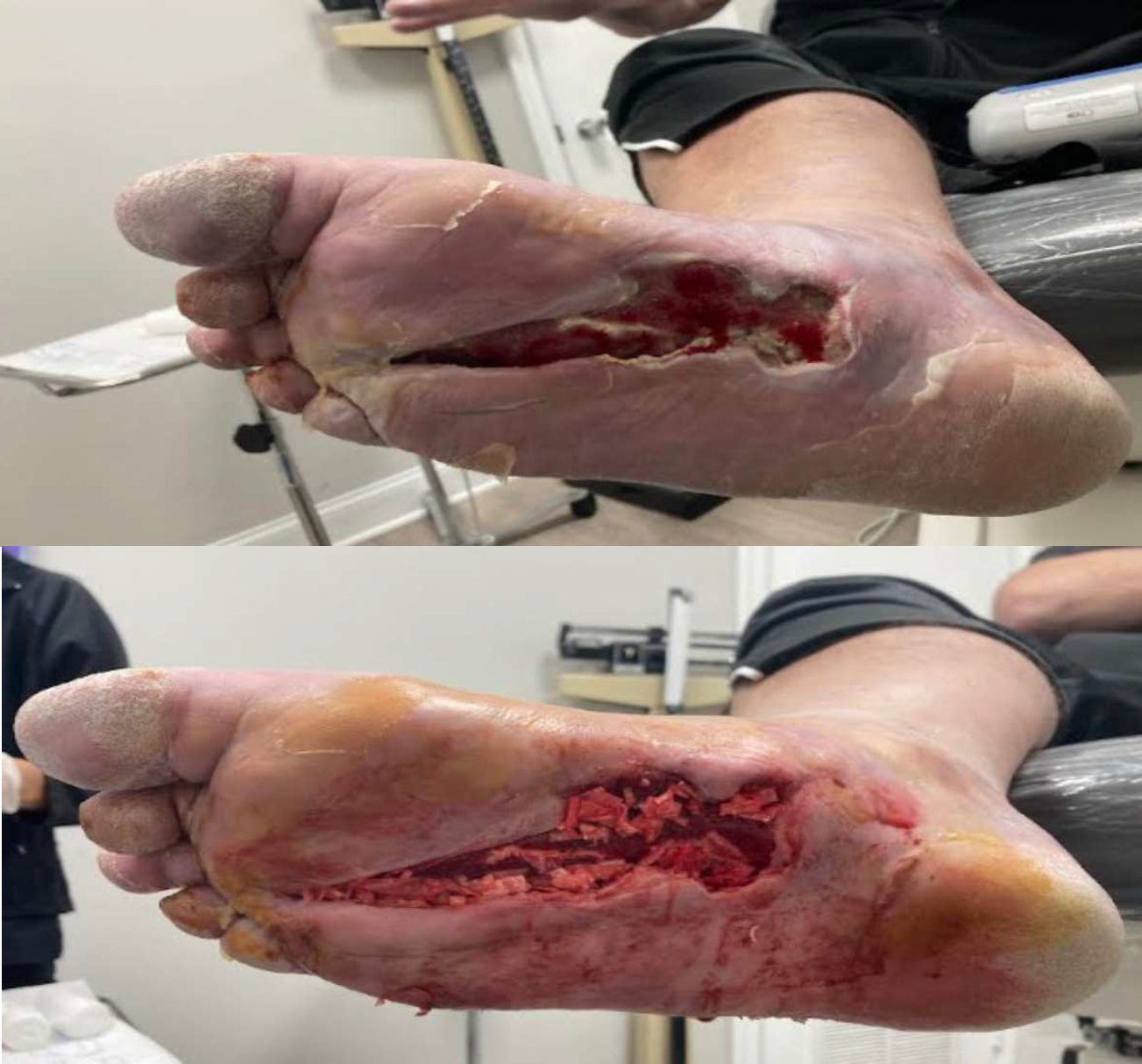
Kerecis Application
Antibiotic beads were removed and excisional debridement was performed to deep, healthy granulation tissue and tendon/muscle up to the flexor tendon sheath proximally. MariGen Micro and MariGen fenestrated were applied with wound VAC therapy. A significant improvement in wound depth was noted 21 days after the first application with no sign of local infection. Continued with weekly wound VAC changes, debridement and 2 additional MariGen applications. At 5 months an advanced skin flap was performed.
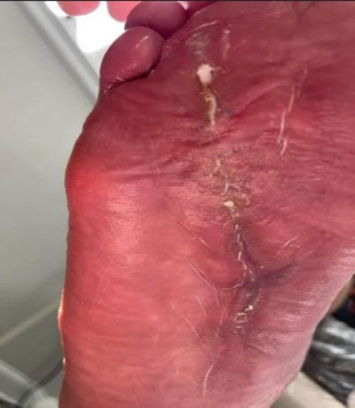
Final Outcome
3 weeks after skin flap and 3 MariGen applications, the surgical incision was completely healed and the patient returned to normal activities.
Need more information?
From the town of Ísafjörður in northwest Iceland, Kerecis develops, manufactures, and distributes patented fish-skin medical devices that support soft tissue regeneration in the body, with regulatory clearance in the United States, Europe, and beyond.