Because no known viral transfer risk exists between North-Atlantic cod and humans, the Kerecis patented fish skin is only gently processed and retains its similarity to human skin. Most skin substitutes products are based on tissues of human and porcine origin. These are not ideal substitutes because heavy processing is needed to eliminate the risk of disease transmission. This harsh, anti-viral treatment removes most of the material’s natural components, making it dissimilar to human skin.
Key Benefits
Structure similar to human skin
No cultural or religious barriers to clinician/patient acceptance
Easy to use, with larger, thicker sheets
No known risk of viral disease transfer
Supports cell ingrowth and vascular ingrowth
Similarity in structure to human skin
Some skin substitutes products are based on tissues of human and porcine origin. Mammalian tissue carries the risk of disease transmission to humans that is nonexistent from the Atlantic cod to humans. Regulatory bodies have strict requirements on tissues from farm animals including viral inactivation methods involving treatment with detergents that remove lipids from the tissues and denature the native structure leaving behind only the most insoluble collagens. Products from human tissues like skin and dehydrated human amnion/chorion membrane call for extensive use of antibiotics to reduce bioburden. The intact fish skin graft is not subject to this harsh treatment, leaving a more naturally intact product with its associated benefits.
Caught in the pristine waters of North Atlantic Ocean
The fish are caught in the pristine waters of North Atlantic Ocean off the township of Isafjordur, on the northwest coast of Iceland. Each and every batch of raw materials is tracked to ensure product quality. The fish skin is processed using a proprietary method that preserves its structure and lipid composition.
Intact fish skin has been cleared by the FDA and European regulatory authorities for wound management. The product is undergoing registration at multiple regulatory authorities around the world.
Kerecis is pioneering the use of intact fish skin in the globally expanding cellular-therapy and regenerative-medicine markets. The unique products from the company’s patented technologies help protect the body’s own tissues and provide the ideal environment to help facilitate tissue regeneration.
Product Configuration
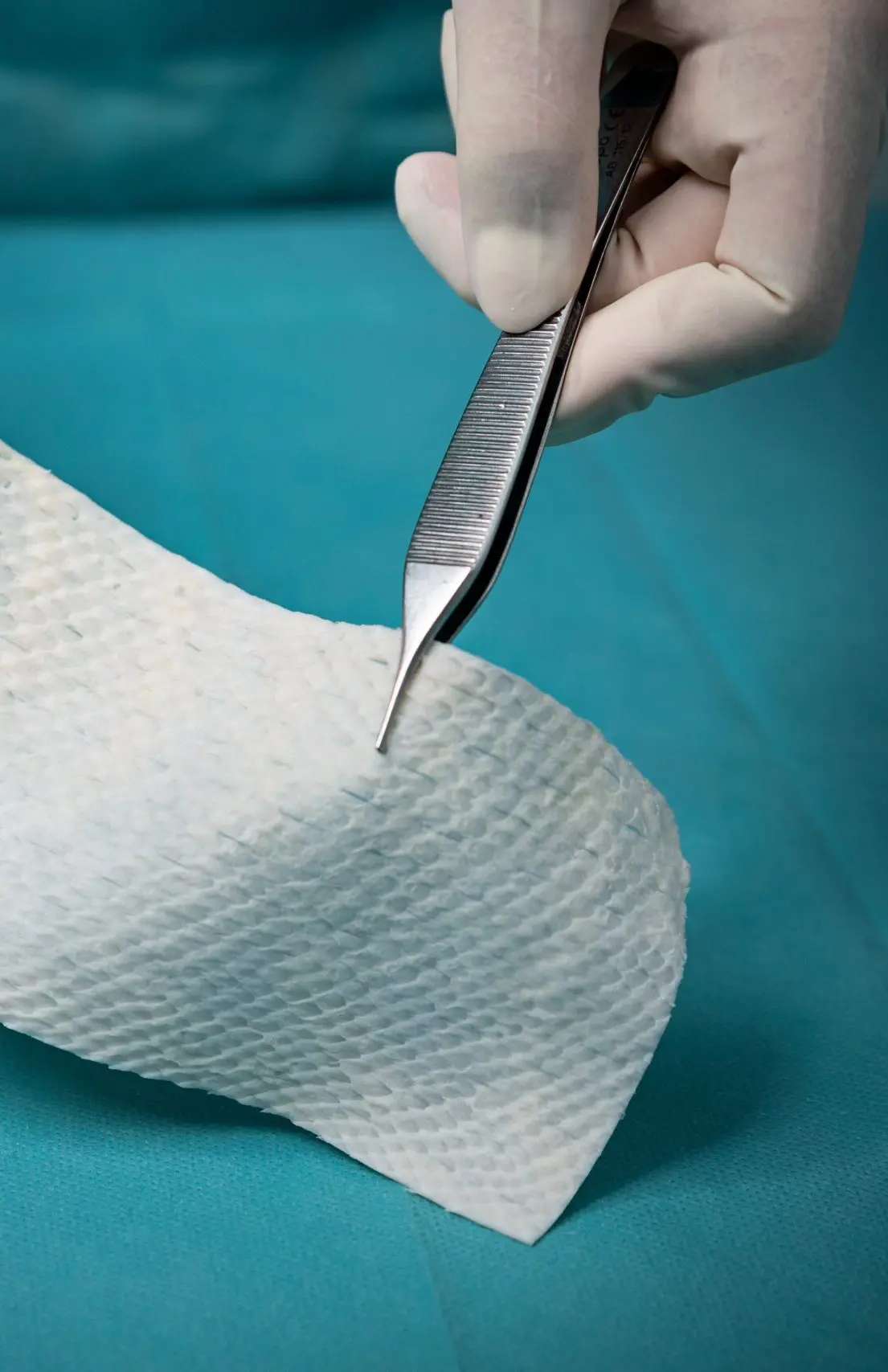
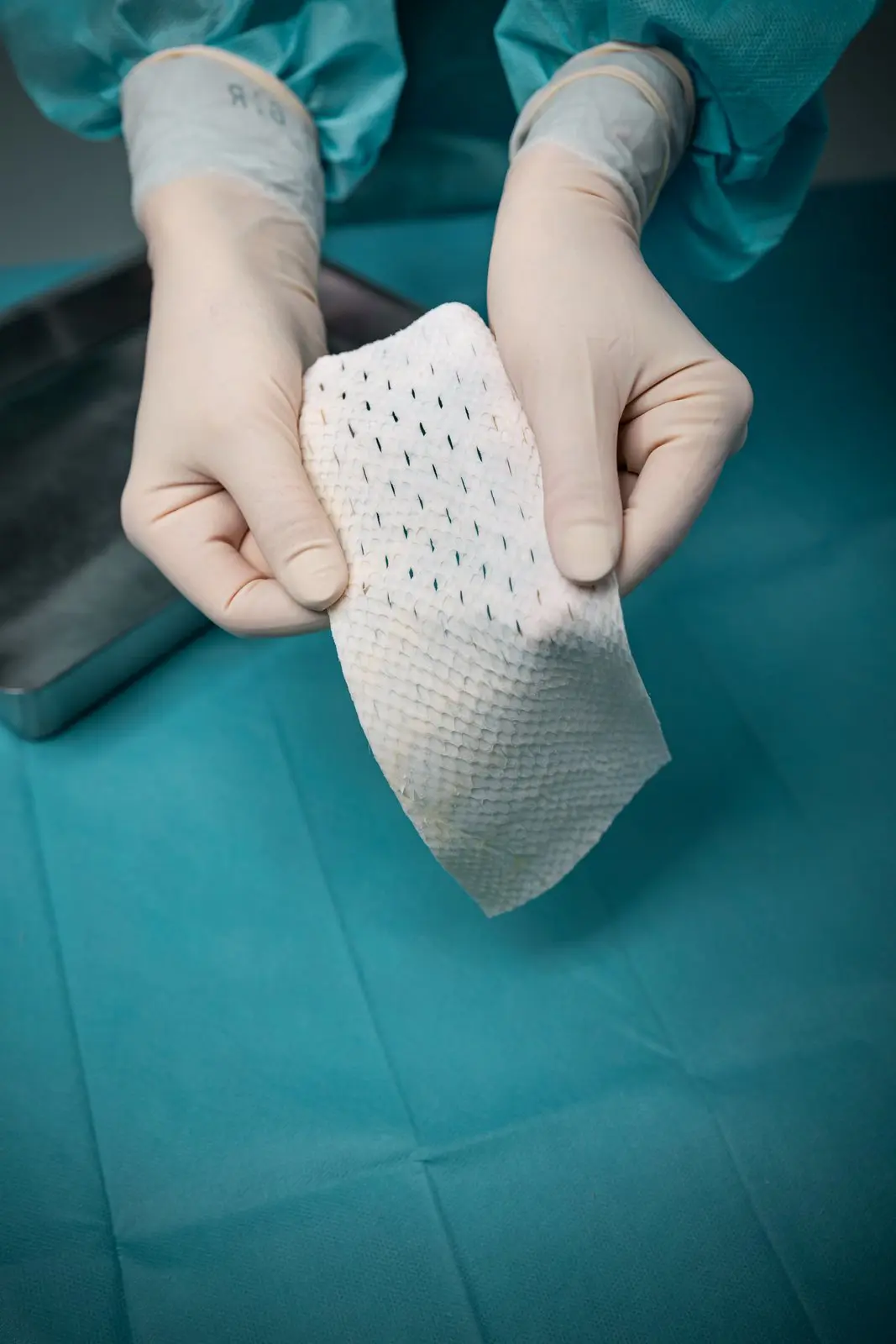
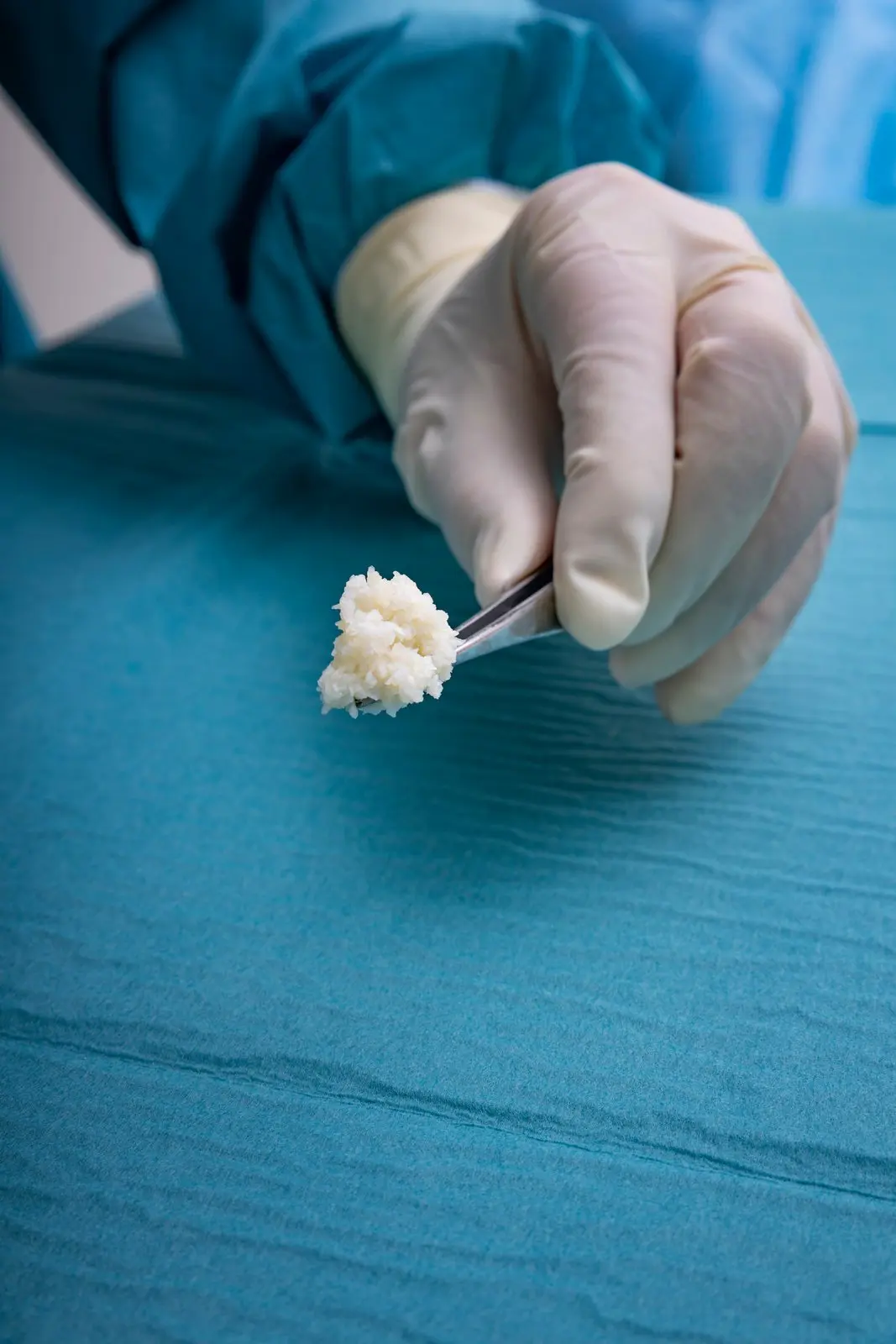
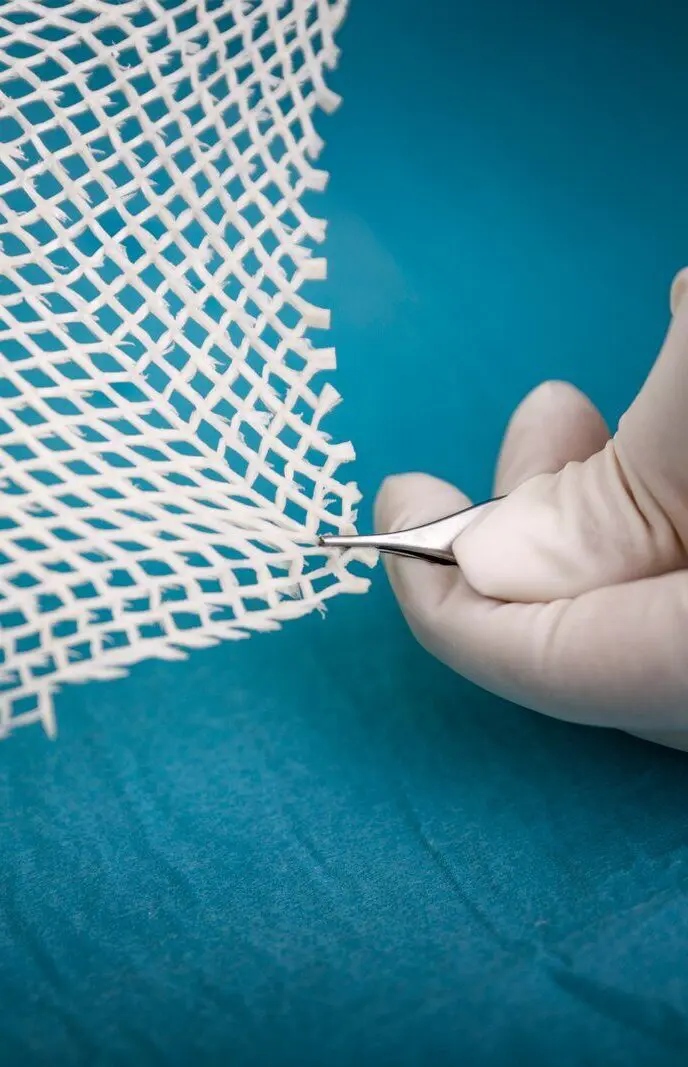
The four Hallmarks of Kerecis Tissue Regeneration
The four Hallmarks of Kerecis Tissue Regeneration
Intact molecular organization
Natural Mechanical Properties
Preserved Molecular Content
Three dimentional structure
Intact molecular organization
Natural Mechanical Properties
Preserved Molecular Content
Intellectual Property
Kerecis has been awarded multiple patents protecting the core technology in the U.S. and other countries, and several more applications are pending. Kerecis is committed to building a substantial patent portfolio protecting the company´s intellectual property.
Need more information?
From the town of Ísafjörður in northwest Iceland, Kerecis develops, manufactures, and distributes patented fish-skin medical devices that support soft tissue regeneration in the body, with regulatory clearance in the United States, Europe, and beyond.
