Symposium on Advanced Wound Care, Denver, Colorado, May 3, 2013— Kerecis today announced that its recent clinical trial found no allergenic reactions to its fish-skin-composed MariGen Omega3 technology. In the trial, 40 patients were tested for allergic reactions after having their skin challenged with MariGen Omega3. Kerecis made the announcement at the Symposium on Advanced Wound Care in Denver, Colorado.
Kerecis is a medical device company focused on the treatment of tissue damage with Omega3-based regenerative technologies. The company is developing a range of tissue-regeneration products based on the company’s MariGen Omega3 technology. The most advanced product is MariGen Wound, which is indicated for the management of chronic wounds, including diabetic, vascular and other hard-to-heal wounds.
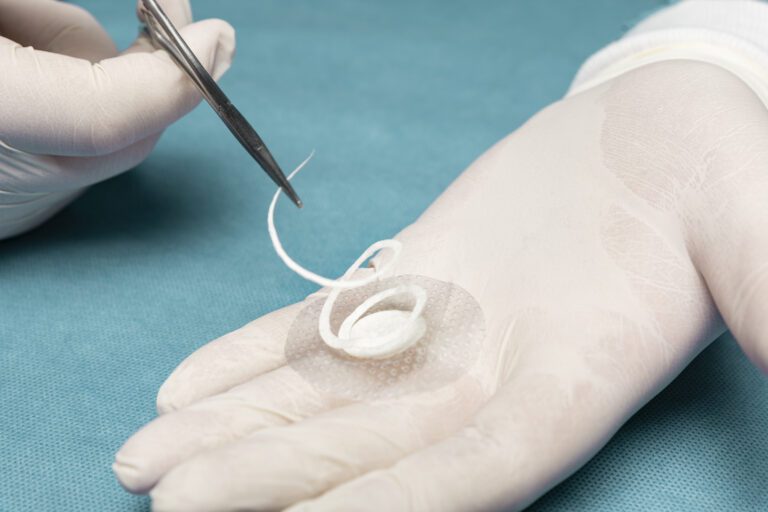
Products based on the MariGen Omega3 technology are intact, decellularized fish skin sheets that have had all cells and antigenic materials removed.(Fish skin is largely made from the same material as human skin, with the addition of Omega3 polyunsaturated fatty acids.)
MariGen Omega3 products are applied to areas of tissue damage where they recruit the body’s own cells, are incorporated into the wound, and ultimately are converted into functional, living tissue. Patents are pending for the MariGen Omega3technology in several countries around the world.
Fish allergies are caused by the GadC molecule, which plays a role in fish muscle movements. The clinical trial demonstrated that no GadC molecules are found in fish skin, which is the basic ingredient of the MariGen Omega3 products.
Comments:
Dr. Baldur Tumi Baldursson (PhD) (MD), Kerecis Medical Director:
“This 40-patient clinical study further demonstrates the safety of the MariGen Omega3 tissue-regeneration technology. More than 100 patients have now participated in our clinical studies and no one has demonstrated any allergenic or otherwise adverse reaction to the material.”
Dr. Hilmar Kjartansson (MD), Kerecis Director for Clinical R&D:
“The allergy study is one of two studies that the U.S. Federal Drug and Food Agency (FDA) required us to execute before clearing MariGen Omega3 for use as a wound-treatment product. We are pleased that we are now half-way through the FDA-required program, and plan to have regulatory clearance before the end of the year.”
For further information, contact:
G. Fertram Sigurjonsson, Founder and CEO, Kerecis.
Phone (Iceland) +354 8494960 / (U.S.) (703) 879-6535
E-mail: gfs@kerecis.com