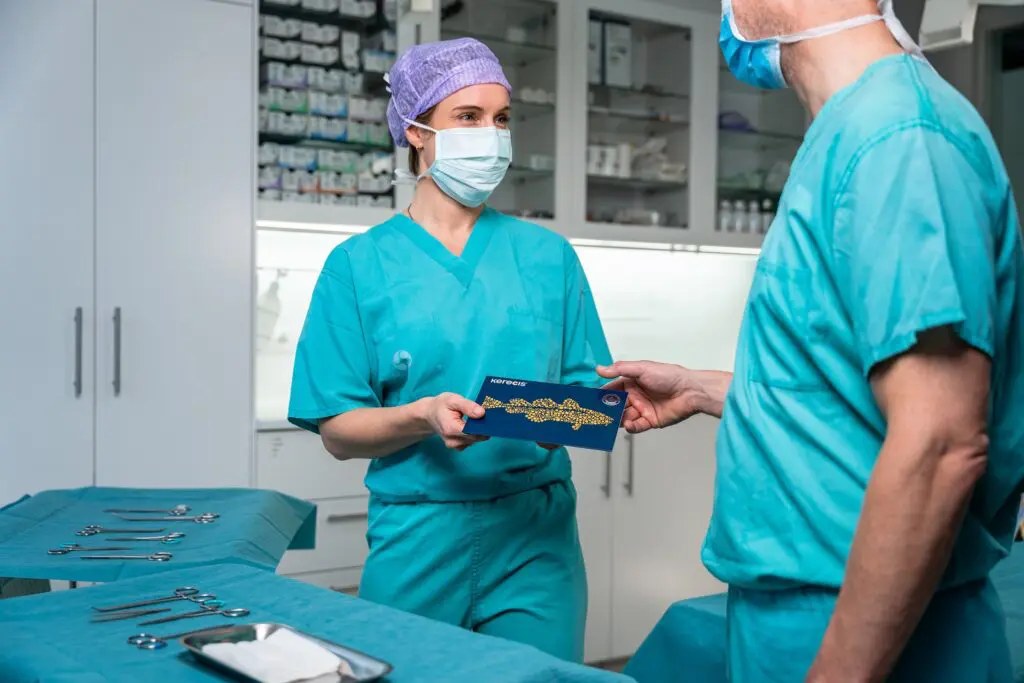
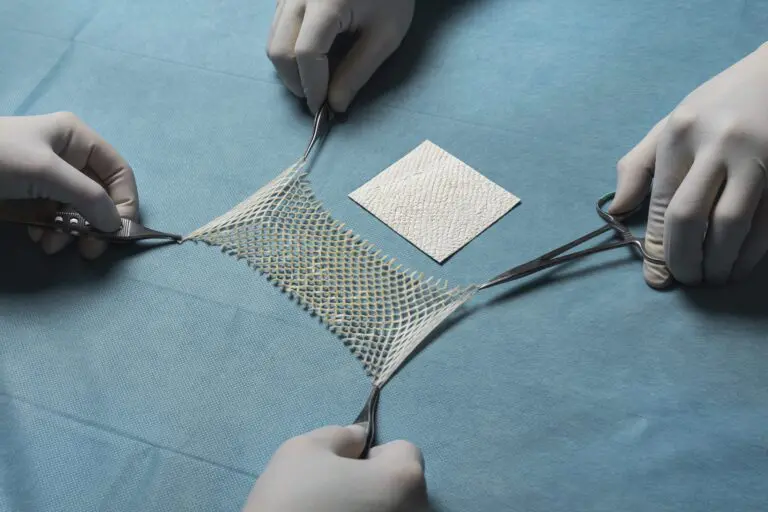
Arlington, VA, and Reykjavik, Iceland – September 9, 2025 – Kerecis, the company pioneering the use of sustainably sourced fish skin and fatty acids in cellular therapy and tissue regeneration, announced four new sizes of its SurgiClose Silicone product line today.
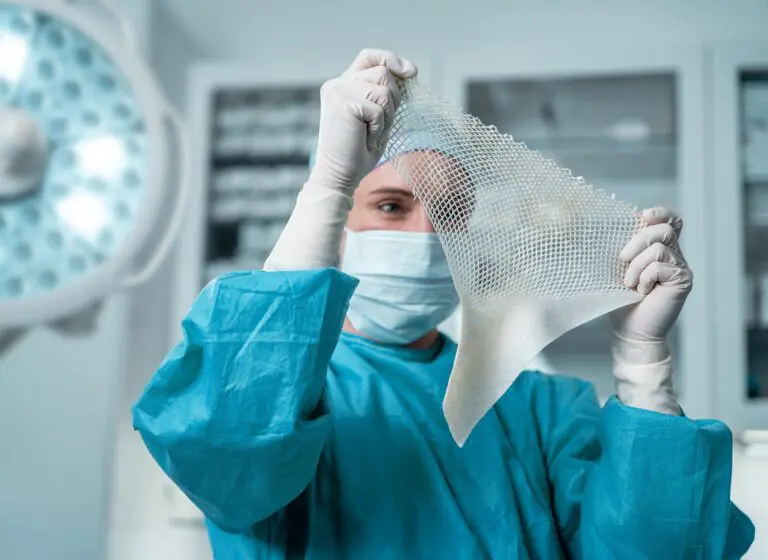
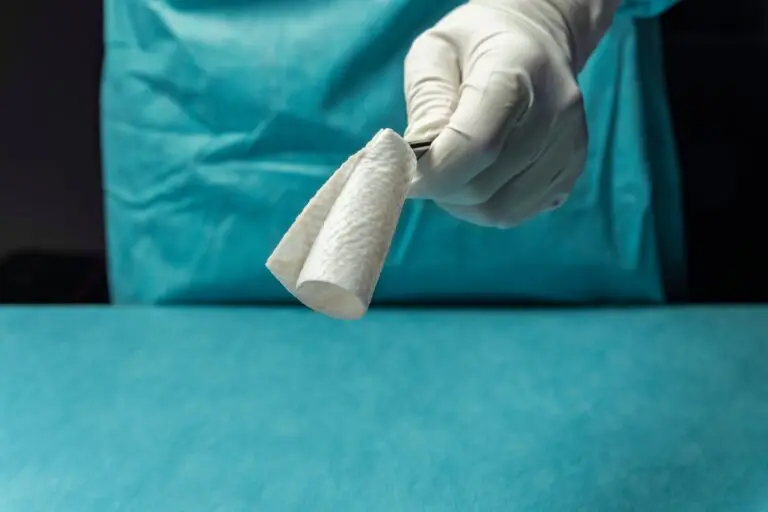
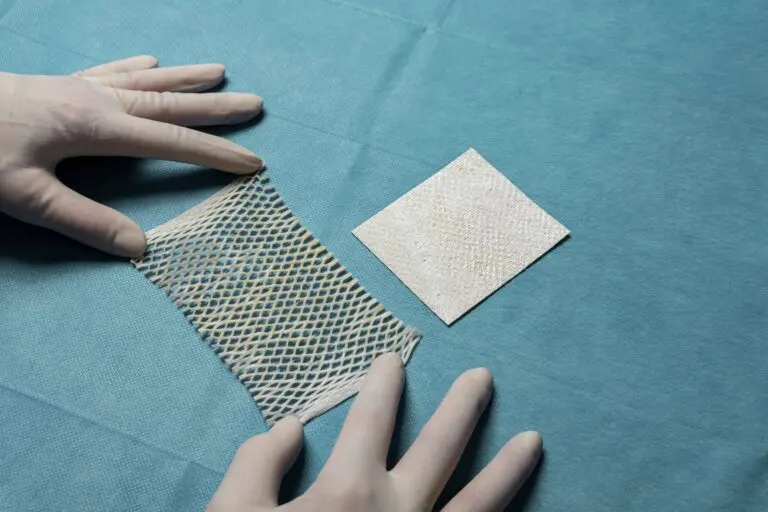
The expanded size range builds on the success of Kerecis’ newest product line for the operating room, SurgiClose Silicone, which combines intact fish-skin technology and a non-adhesive silicone layer to efficiently manage exudate and wound moisture.
The four new sizes will accommodate smaller wounds, ranging from a 3×3 cm bordered product to a 10×10 cm borderless version. With this expanded range, SurgiClose Silicone’s powerful combination of non-adhesive silicone layer and intact fish-skin technology will support wound healing for even more patients, while simplifying treatment for more care teams.