Arlington, VA & Reykjavik, Iceland – July 23, 2025 – Kerecis, the company pioneering the use of fish-tissue in cellular therapy and tissue regeneration, will debut its latest innovation, SurgiBind® Tendon Protect, at the American Podiatric Medical Association’s (APMA) Annual Scientific Meeting in Grapevine, Texas, July 24-27, 2025. The new biologic graft is designed for tendon procedures where no significant tissue loss is present and will be spotlighted during educational and interactive sessions throughout the conference.
APMA 2025
As part of its presence at APMA 2025, Kerecis will exhibit at Booth #805 and host a clinical education event, “Taste of Iceland,” on Friday, July 25, from 7:00 to 9:00 PM at Mission Plaza, Gaylord Texan Resort. During the session, Dr. James Cottom, DPM, will present “Gliding into Enhanced Tendon Outcomes with Intact Fish Skin,” a talk exploring the clinical use of SurgiBind® Tendon Protect in tendon repair procedures. Attendees will also have the opportunity to learn more about the broader application of intact fish-tissue grafts while sampling traditional Icelandic cuisine.
Designed for Tendon Protection and Surgical Efficiency
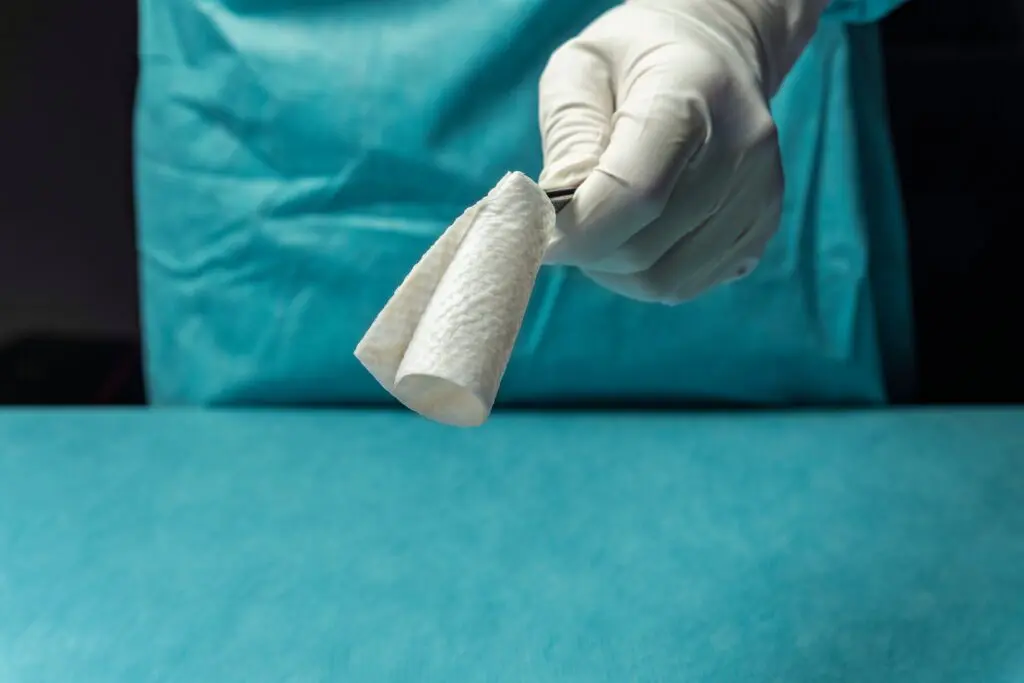
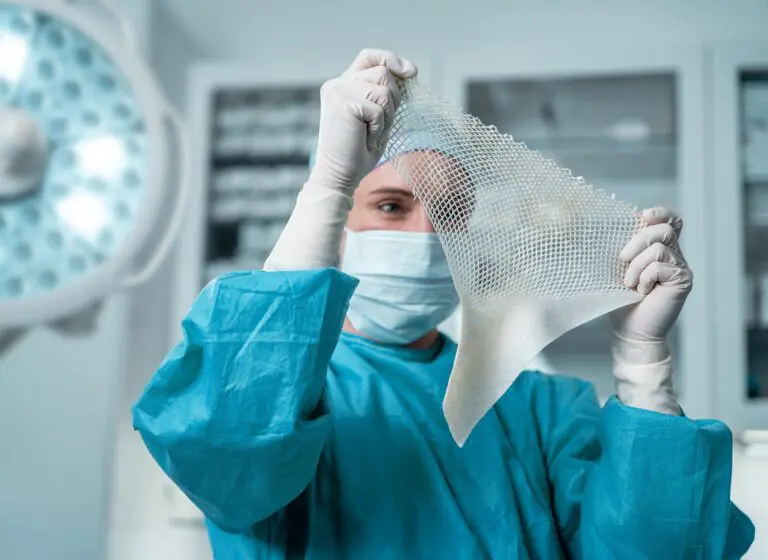
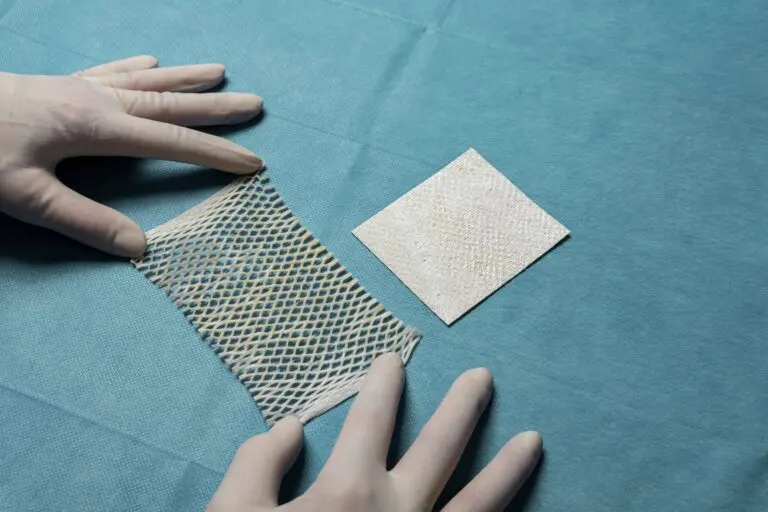
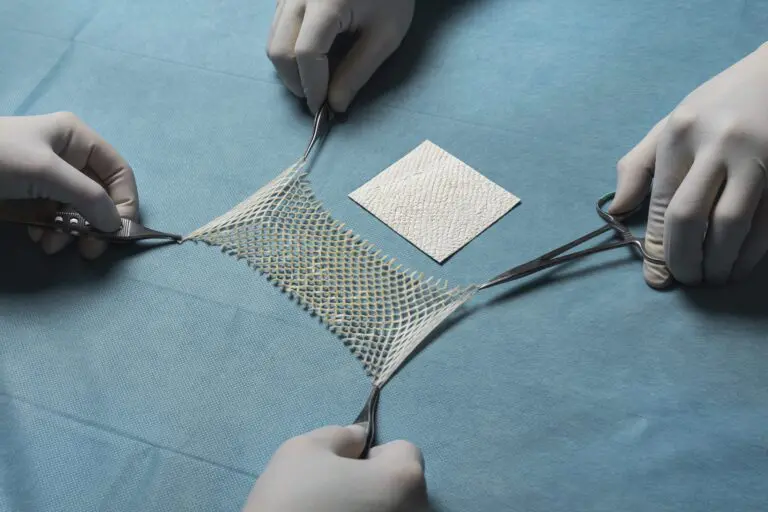
Each year, an estimated 500,000 tendon procedures are performed in the United States, many of which aim to preserve or restore movement following tendon injury. SurgiBind® Tendon Protect is intended to be wrapped around a repaired tendon during surgery and is cleared for implantation to reinforce soft tissue where weakness exists. The product is a solid, intact fish-tissue based graft, sustainably sourced from North Atlantic cod, and is bioresorbable, non-crosslinked, and designed to be sutured and positioned with ease in the surgical field.