Kerecis GraftGuide® Products
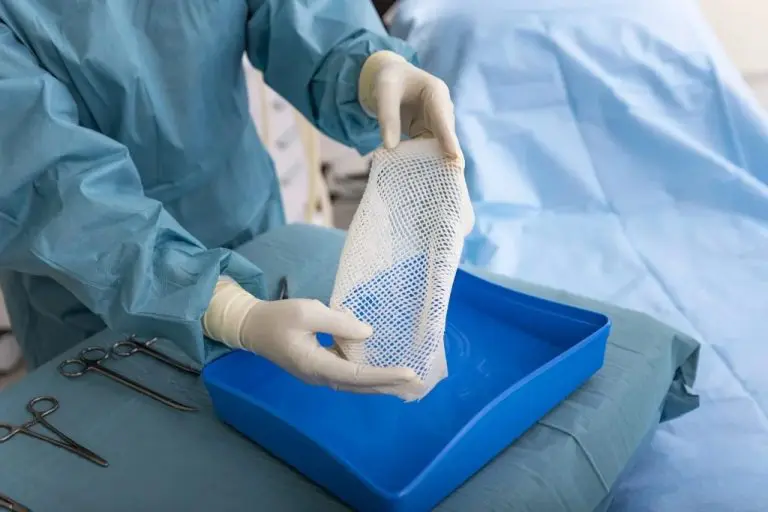
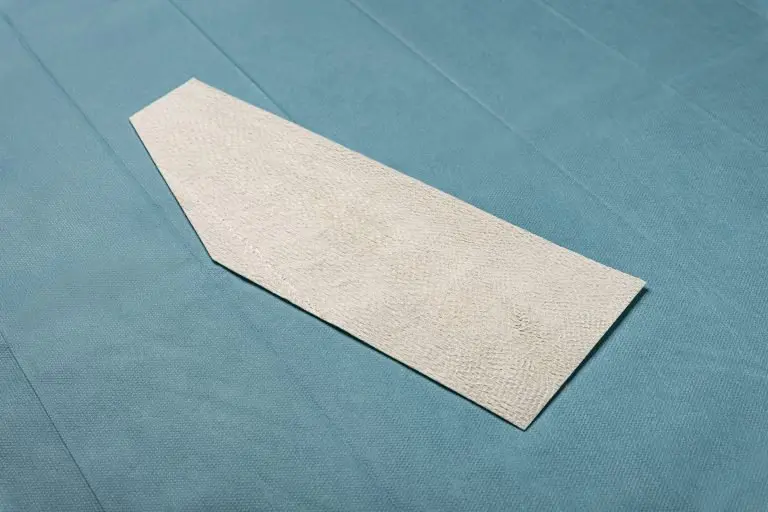
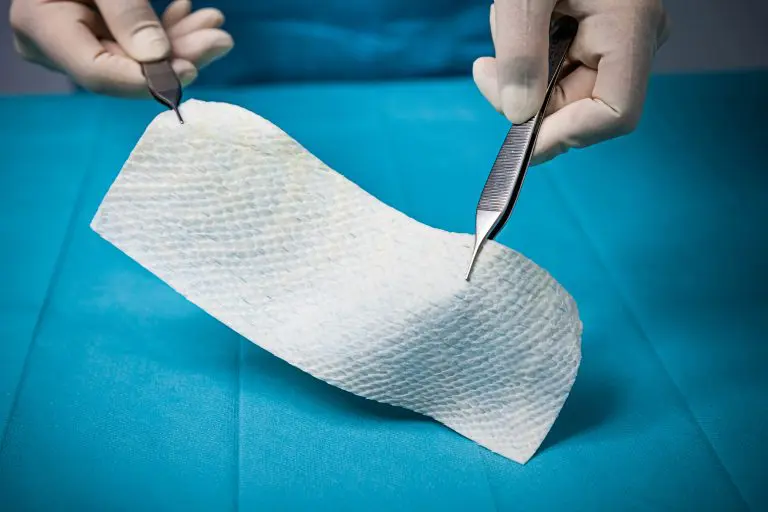
Kerecis GraftGuide is intact fish skin especially developed for the management of burn wounds and donor sites.
The Kerecis fish-skin grafts contain fat, protein, elastin, glycans and other natural skin elements and are supplied in multiple shapes, variants and sizes.
The product classifies as a medical device and consists of a full thickness fish-skin that has been processed using Kerecis’ proprietary EnviroIntact™ method.

GraftGuide® Mano
The intact fish-skin graft in the shape of the hand to easily allow application on its complex 3D structure. Available in two sizes, medium and large, and in either left or right versions, for use in palmar or dorsum applications. Designed to reduce operation time when managing hand burn injuries.
GraftGuide Meshed 2:1
2:1 pre-meshed intact fish-skin graft designed to expand and cover larger wounds. Provides a viable clinical and economic solution for large wounds.
Our Vision is to extend life by supporting the body’s own ability to regenerate.
Largest RCT on DFUs with Exposed Bone or Tendon Brings New Hope for Patients
Groundbreaking study fills critical gap in evidence-based treatment options for DFUs with exposed structure.

Benefits of Using GraftGuide Products
Accelerated Recovery
Decreased Length of Stay
Coverage for Large Areas
Enhanced Patient Comfort
See how Kerecis Helped Burn Survivor Pétur Oddsson
Indication
- Partial and full thickness wounds.
- Trauma wounds, including abrasions, lacerations, second-degree burns, skin tears.
- Surgical wounds, including donor sites, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence.

Other Kerecis Products for Operating Room Use
Need more information?
From the town of Ísafjörður in northwest Iceland, Kerecis develops, manufactures, and distributes patented fish-skin medical devices that support soft tissue regeneration in the body, with regulatory approvals in the United States, Europe, and beyond.
Important Information
Baldursson, B. T. et al. Healing rate and autoimmune safety of full-thickness wounds treated with fish skin acellular dermal matrix versus porcine small-intestine submucosa: a non-inferiority study. Int. J. Low. Extrem. Wounds 14, (2015).
Magnusson, S. et al. Decellularized fish skin: characteristics that support tissue repair. Laeknabladid 101, 567–573 (2015)
Shupp, J. W. et al. Fish Skin Compared to Cadaver Skin as a Temporary Covering for Full Thickness Burns: An Early Feasibility Trial. Poster presented at: AMUS 2020 Annual Meeting (2020).
Stone R 2nd, Saathoff EC, Larson DA, et al. Accelerated Wound Closure of Deep Partial Thickness Burns with Acellular Fish Skin Graft. Int J Mol Sci. 2021;22(4):1590.
Wallner, C. et al. A Comparison of Intact Piscine Skin, Split-thickness Skin Graft, and Lactic Acid Membrane in Treating Superficial and Deep Burn Wounds Following Enzymatic Debridement, J Burn Care Res. 42 (Suppl 1): 125-126 (2021).