Who We Are
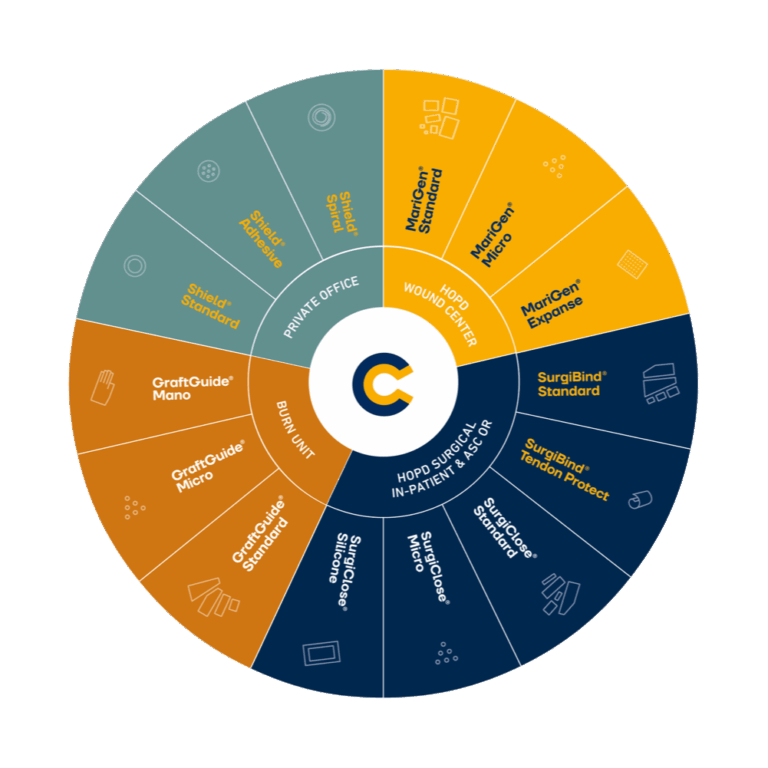
From the town of Isafjordur in northwest Iceland, Kerecis develops, manufactures, and sells patented fish-skin soft tissue regeneration products that have regulatory approval in the United States, Europe and several other jurisdictions.
We provide our customers with innovative digital tools for managing their business, including artificial intelligence (AI) to support insurance billing.
We have regional headquarters in the United States, Iceland, and Germany with more than 620 employees including a 260-person US salesforce.
Kerecis is part of Coloplast A/S.