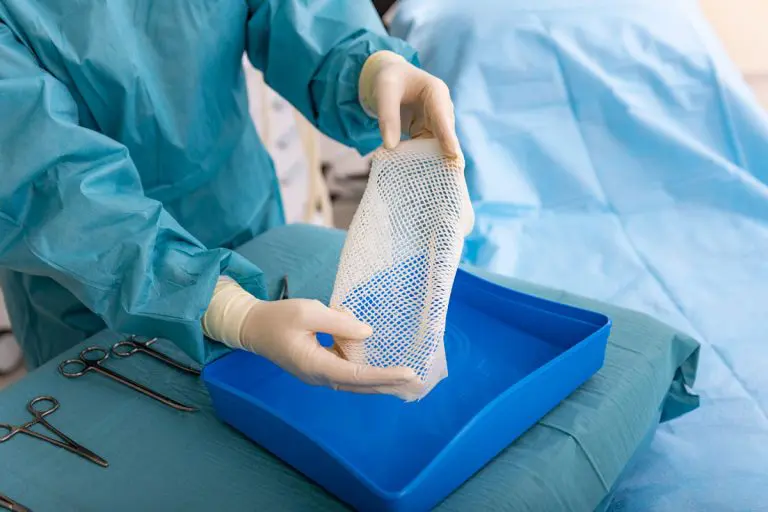
Introducing SurgiBind® Tendon Protect
Each year, an estimated 500,000 tendon procedures are performed in the United States, many of which aim to preserve or restore movement following tendon injury.
SurgiBind Tendon Protect is indicated for the management and protection of tendon injuries in which there has been no substantial loss of tendon tissue. Now available in sizes that are ideal for many upper and lower-extremity tendon procedures, SurgiBind helps manage and protect newly-repaired tendons by providing a covering around the tendon after repair.
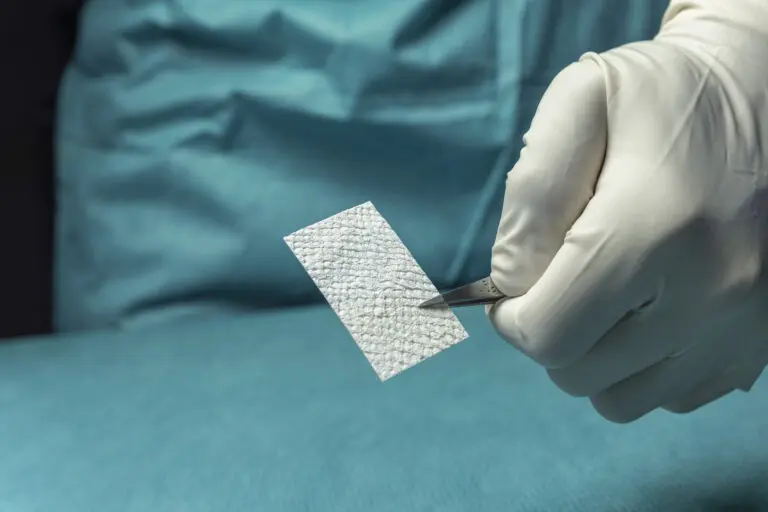
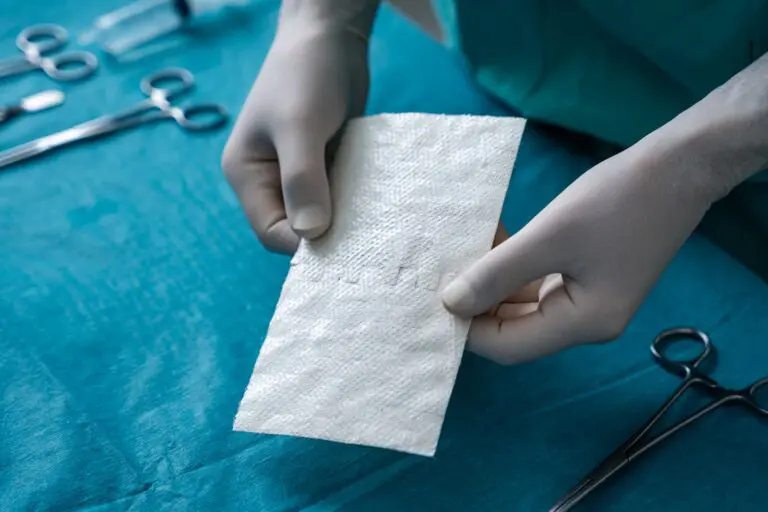
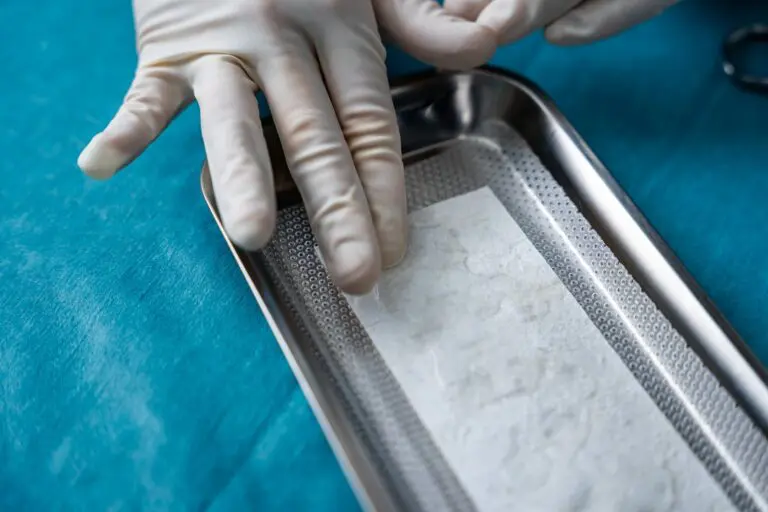
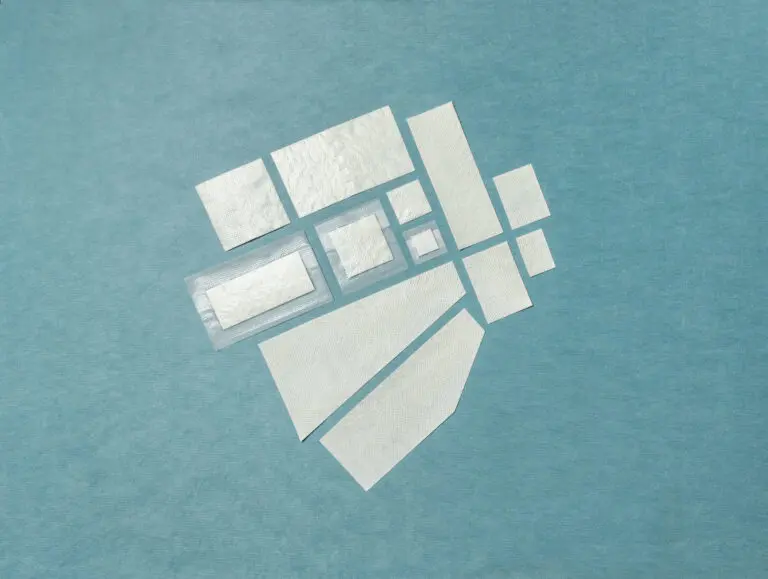
SurgiBind is intact fish skin intended for surgical implantation. The product can be used to reinforce soft tissue where weakness exists. The Kerecis fish skin graft is supplied in multiple shapes, variants, and sizes and contains natural skin elements and structure. The product classifies as a medical device and consists of a full thickness fish skin that has been processed using Kerecis’ proprietary EnviroIntact™ method.
SurgiClose is an intact fish skin graft used for supporting tissue regeneration on surgical, traumatic and acute wounds. SurgiClose Silicone provides a breakthrough balance of protection, breathability, and stability with a combination of fenestrated fish skin and a highly-refined, porous silicone contact layer.
Building on the success of our newest product line for the operating room, we’re excited to announce four new sizes of SurgiClose® Silicone. These new sizes will accommodate smaller wounds ranging from a 3×3 cm bordered product to a 10×10 cm borderless version. With this expanded range, our powerfull combination of non-adhesive silicone layer and intact fish-skin technology can now support wound healing for even more patients while simplifying treatment for care teams.
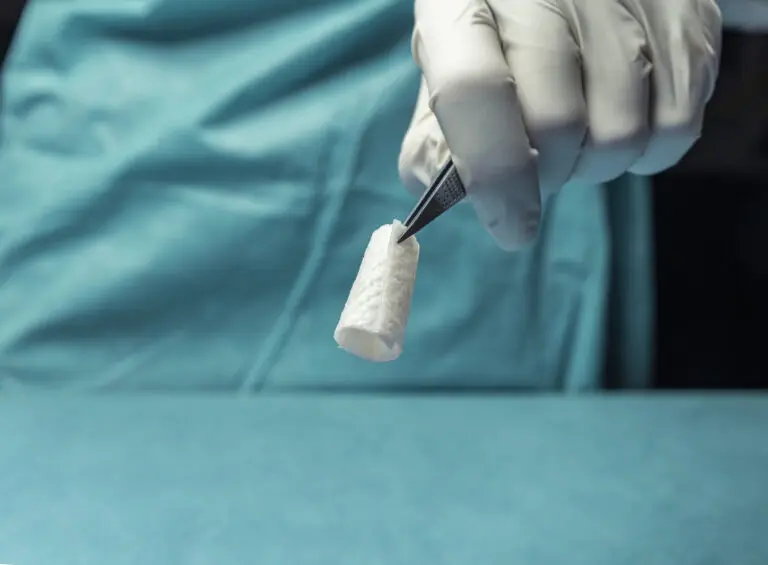
SurgiBind® Tendon Protect
SurgiBind Tendon Protect is a solid sheet of intact fish skin intended for the protection and management of newly repaired tendons.
SurgiClose® Silicone
SurgiClose Silicone provides a breakthrough balance of protection, breathability, and stability with a combination of fenestrated fish skin and a highly-refined, porous silicone contact layer.
SurgiClose® Micro
Intact fish skin graft is cut into smaller sheets using a process that preserves the integrity and composition of the material.
SurgiBind® Fenestrated
Intact fish skin graft is fenestrated to allow for easier flow through of liquids
SurgiClose® Meshed 2:1
2:1 pre-meshed intact fish skin graft is designed to expand and cover larger wounds. It provides a viable clinical and economic solution for large wounds.
Largest RCT on DFUs with Exposed Bone or Tendon Brings New Hope for Patients
Groundbreaking study fills critical gap in evidence-based treatment options for DFUs with exposed structure.

Benefits of Using SurgiClose and SurgiBind
Reduced Procedure Time
Enhanced Wound Healing
Versatile Applications
Built on Sustainability
See how Kerecis saved this patient’s life with Necrotizing Fasciitis
Other Kerecis Products
Indication for SurgiClose
- Trauma Wounds
- Abrasions
- Lacerations
- Skin Tears
- Surgical Wounds
- Donor site/grafts
- Post-Mohs surgery
- Post-laser surgery
- Podiatric
- Dehiscence
Indication for SurgiBind
- For implantation to reinforce soft tissue where weakness exists, in patients requiring soft tissue repair, or reinforcement in plastic or reconstructive surgery
Indication for SurgiBind Tendon Protect
- SurgiBind Tendon Protect is indicated for the management and protection of tendon injuries in which there has been no substantial loss of tendon tissue
Need more information?
From the town of Ísafjörður in northwest Iceland, Kerecis develops, manufactures, and distributes patented fish-skin medical devices that support soft tissue regeneration in the body, with regulatory clearance in the United States, Europe, and beyond.