About The Product
MariCell FOOTGUARD is a topical dermatology product formulated for use on the outermost layers of the skin, particularly on thick skin, calluses, and cracked heels.
MariCell FOOTGUARD is a topical dermatology product formulated for use on the outermost layers of the skin, particularly on thick skin, calluses, and cracked heels.


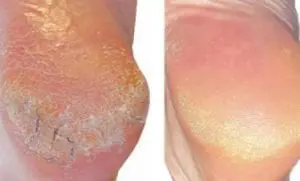
Day 1 vs Day 17
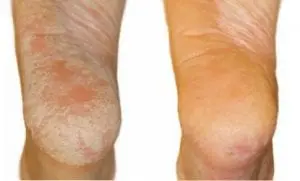
Day 1 vs Day 19
The outermost layer of the skin consists of 15 to 20 layers of dead cells that have dried out and died. This is the corneal layer of the skin. Between the dried cells is the intercellular substance, which is rich in fatty acids that keep the structure intact and watertight.
In the case of extreme skin dryness, the corneal layer of the skin loses the fatty acids, dries out, and no longer protects the living cells below. This causes skin tightness, inflammation, itching, and callus formation that can become cracked and painful.
Maricell™ Footguard™ triple action formula removes dead skin from the feet and hydrates remaining skin to prevent build up of dead skin (calluses) again.
From the town of Ísafjörður in northwest Iceland, Kerecis develops, manufactures, and distributes patented fish-skin medical devices that support soft tissue regeneration in the body, with regulatory approvals in the United States, Europe, and beyond.