About the Product
MariCell ™ SMOOTH is a topical cream designed for the treatment of the outermost layer of the skin.
MariCell ™ SMOOTH is a topical cream designed for the treatment of the outermost layer of the skin.


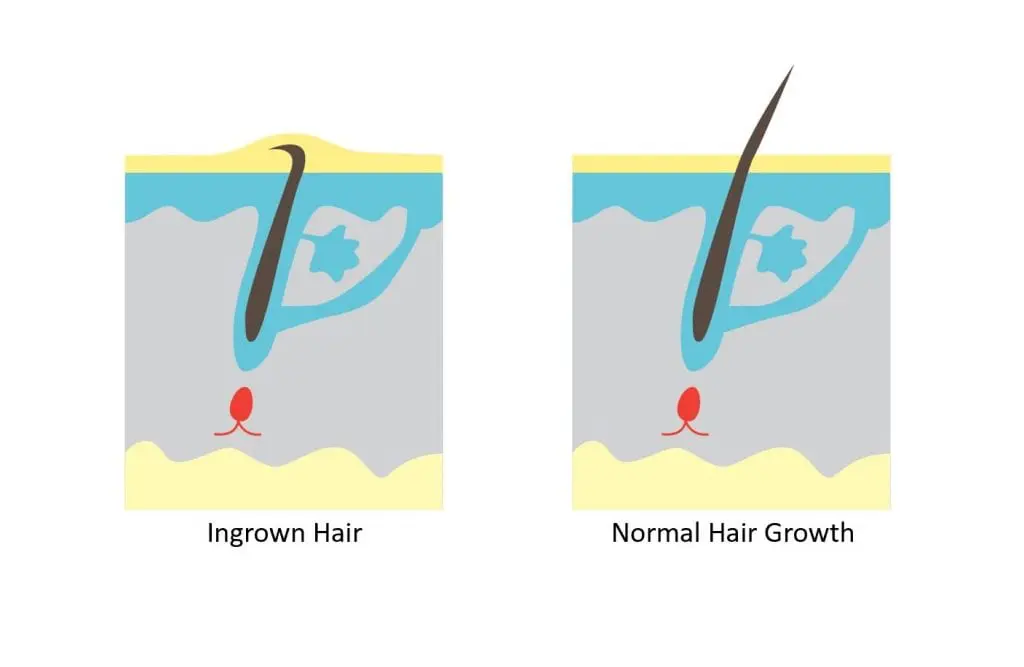
The outermost layer of the skin consists of 15 to 20 cell layers of dead cells that have dried out and died. This is the corneal layer of the skin. Between the dried cells is the intercellular substance, which is rich in fatty acids that keep the structure intact and watertight.
Various complications can cause the formation of skin bumps. Complications include hair follicles becoming blocked by skin-fat plugs and skin contracting after
shaving (pseudofolliculitis barbae). Some skin is too dense, blocking the growth of tiny body hairs from hair follicles causing a condition called chicken skin (keratosis pilaris). The results are rough patches of small, acne-like bumps, usually on the arms, thighs and buttocks. The bumps are usually white, sometimes red, and generally don‘t hurt or itch.
Maricell™ Smooth softens the outermost layer of the skin making it easier for the tiny body hairs to grow and also softens the skin bumps so that they can easily be rubbed off
From the town of Ísafjörður in northwest Iceland, Kerecis develops, manufactures, and distributes patented fish-skin medical devices that support soft tissue regeneration in the body, with regulatory clearance in the United States, Europe, and beyond.