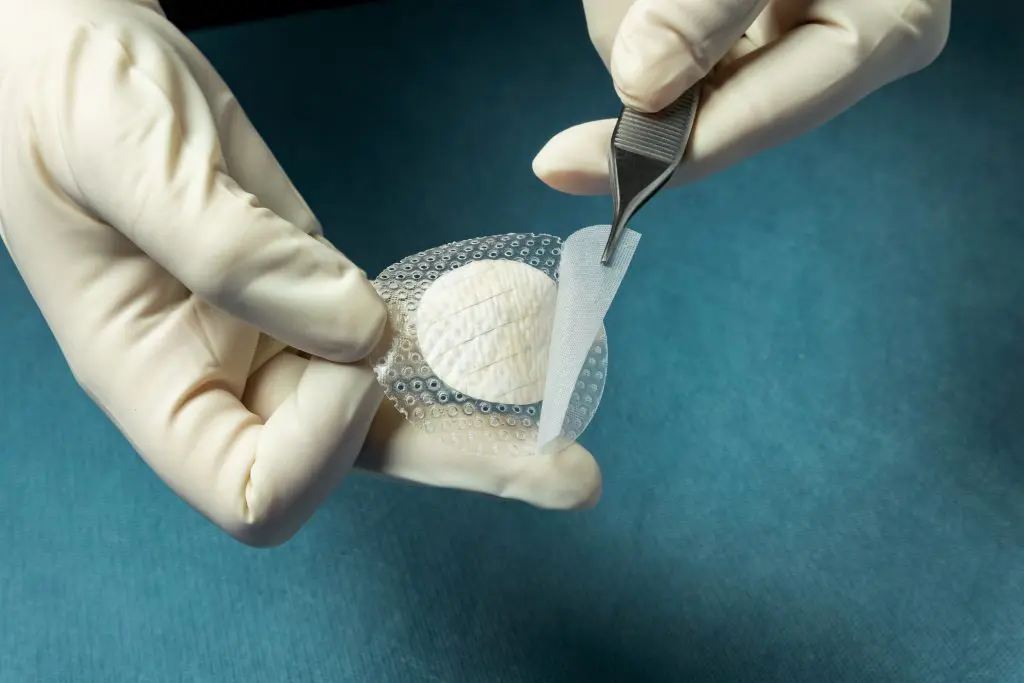
The Kerecis fish-skin grafts contain fat, protein, elastin, glycans, and other natural skin elements and are supplied in multiple shapes, variants, and sizes. The product integrates the Kerecis intact fish skin with a silicone contact layer.
Shield is fenestrated to allow adequate wound drainage for appropriate wound moisture at the wound bed.
The product classifies as a medical device and consists of intact fish skin processed using Kerecis’ proprietary EnviroIntact™ method. The product is not subject to the May 31, 2021, FDA 351 regulation.
Product information
Manufacturer Name
Product Name (s)
Brand Name
Generic Name
HCPCS Code
A2019 HCPCS Code Listed Product Description
FDA Registration
FDA Clearance Date
FDA Clearance Type
First Marketing Date
Unit for Strength
Number of Items per Package
Number of Items per Alternate ID
Units of Volume per Item for all Alt ID’s
| Product Code | Shield Product Description – Size | Total Size cm2 | Billable Units | HCPCS Code | UPC |
|---|---|---|---|---|---|
| 50215I53B0D | Shield Standard – 50 mm Circular, Diameter | 19.64 | 20 | A2019 | 5694310964319 |
| 50215I52B0D | Shield Adhesive – 30 mm Circular, Diameter | 7.07 | 8 | A2019 | 5694310963770 |
| 50215H51B0D | Shield Adhesive – 20 mm Circular, Diameter | 3.14 | 4 | A2019 | 5694310963756 |
| 50215K52B0D | Shield Spiral – 30 mm Diameter Circular | 7.07 | 8 | A2019 | 5694310964364 |
| 50215H50B0D | Shield Adhesive – 15 mm circular, diameter | 1.77 | 2 | A2019 | 694310965187 |
| 50215L54B0D | Shield Spiral – 80 mm circular, diameter | 50.27 | 51 | A2019 | 5694310965217 |
Additional Information
Important Information
Need more information?
From the town of Ísafjörður in northwest Iceland, Kerecis develops, manufactures, and distributes patented fish-skin medical devices that support soft tissue regeneration in the body, with regulatory clearance in the United States, Europe, and beyond.