
Arlington, VA and Reykjavik, Iceland – Oct. 4, 2024 – Kerecis, the pioneer in the use of fish-skin and fatty acids for tissue regeneration and protection, today announced the publication of breakthrough study results in the NEJM Evidence. The study was conducted on a sample of 255 patients at 15 care centers across four countries. The study is the largest randomized controlled trial published to date on the efficacy of using biologic skin substitutes to treat foot ulcers with exposed bone and tendons. The study demonstrates that fish-skin grafts significantly outperform the standard of care in closing University of Texas grade 2 and 3 diabetic foot ulcers.
Kerecis’s presentation of the study results met with an overwhelmingly positive reception during the Symposium on Advanced Wound Care (SAWC) held this month in Las Vegas.
This study – named Odinn as an homage to the Norse god of healing and wisdom – is by far the largest randomized controlled trial (RCT) of its kind because of the number of patients and severity of the wounds studied (University of Texas grades 2 and 3). The findings underscore the efficacy of fish-skin grafts in the treatment of diabetic foot ulcers (DFU), offering new hope and improved outcomes for patients.
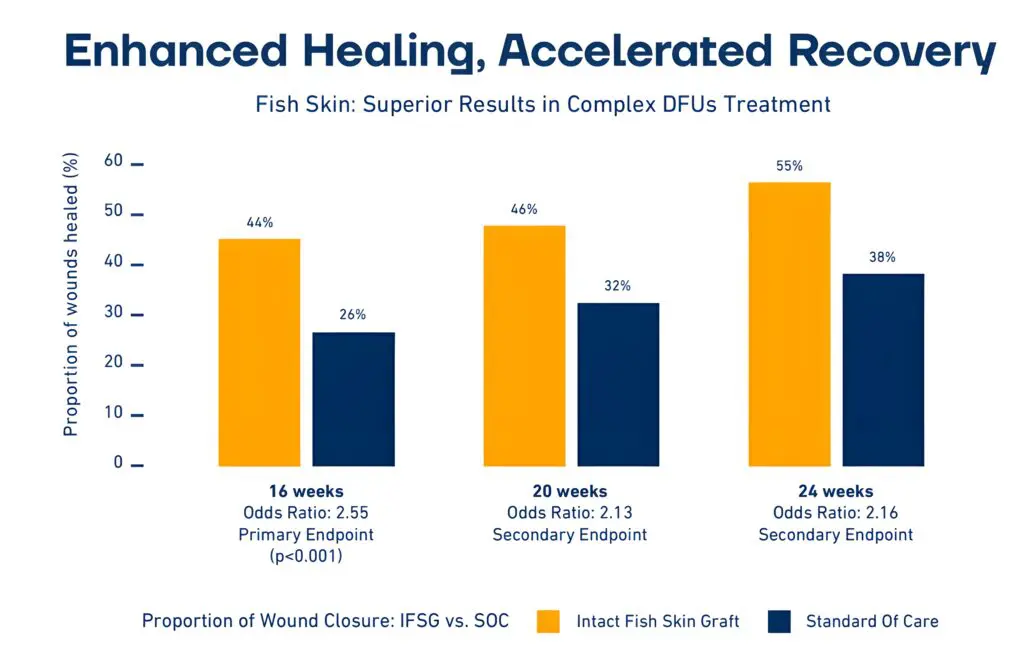
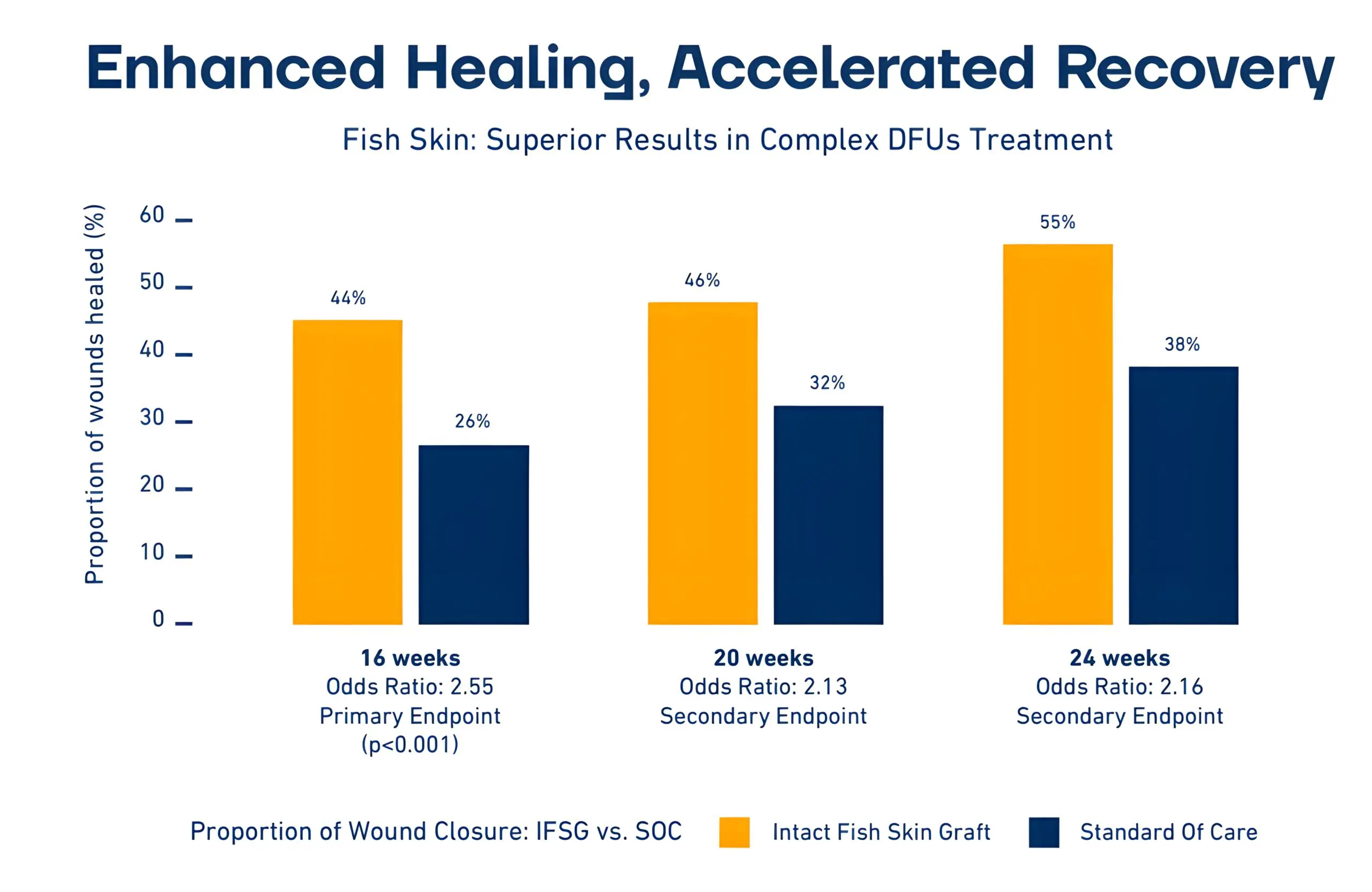
1. Significantly higher healing rates: At 16 weeks, 44% of DFUs treated with Kerecis Fish-skin Grafts had healed, compared with 26.4% using SOC (p<0.001).
2. Sustained superiority: This trend continued, with 55.2% of fish-skin graft-treated wounds healed at 24 weeks versus 37.8% for standard care.
3. Faster healing: In the study, diabetic foot ulcers treated with intact fish-skin graft were associated with faster wound closure time, with the wounds healing approximately two weeks earlier and with a 1.6x (95% CI: 1.07 – 2.36) greater chance of wound closure at any time point.
University of Texas Grade 2 DFUs are serious wounds that penetrate deeply into the foot, reaching the tendon or capsule and involve subcutaneous tissues. Due to their depth and complexity, these ulcers require more intensive treatment. Grade 3 DFUs are even more severe, extending to the bone or joints and posing a higher risk of serious complications, such as infections and amputations. Due to their critical nature, Grade 3 DFUs often require aggressive and specialized treatment.
DFUs are chronic, life-threatening conditions characterized by difficult and delayed healing. The consequences of these wounds are often severe, resulting in amputation and increased mortality rates as well as the clinical and fiscal impact of DFUs on patients’ lives.
The study was funded through a competitive grant from the European Union’s Horizon 2020 research and innovation program.
Kerecis Scientific Advisory Board Chairman John Lantis, MD says the study is truly a landmark. “The Odinn Trial is pivotal in diabetic foot ulcer treatment, especially for the more complex grades 2 and 3 cases. It provides compelling evidence that fish skin grafts could redefine the standard of care. With rigorous methodology and strong results, Odinn paves the way for a promising shift in clinical practice, where fish skin treatment may soon become the go-to option for managing severe diabetic foot ulcers, ultimately leading to better patient outcomes and quality of life.”
Fertram Sigurjonsson, founder and CEO of Kerecis, says “By conducting the largest RCT for complex DFUs that expose bone, tendon, or joints to date, we are breaking new ground in treatment of serious wounds and tissue damage. I am very proud to bring hope to patients who face the highest risks of complications and amputations, showing that innovative solutions can truly transform lives. Our team is committed to bringing our revolutionary treatment to healthcare providers and patients around the world, working toward our mission of making the Kerecis fish-skin the standard of care for serious wounds worldwide.”
Healthcare providers specializing in wound care, podiatry, and diabetic foot care are encouraged to visit www.kerecis.com/publication to learn more about the groundbreaking findings and the efficacy of fish-skin grafts in treating wound and tissue damage.
Media Note: For additional information about this study, information on 50 additional studies on the efficacy and benefits of fish-skin grafts, or to schedule an interview, contact the Media Relations Agency at (952) 697 5220.
From the town of Ísafjörður in northwest Iceland, Kerecis develops, manufactures, and distributes patented fish-skin medical devices that support soft tissue regeneration in the body, with regulatory clearance in the United States, Europe, and beyond.