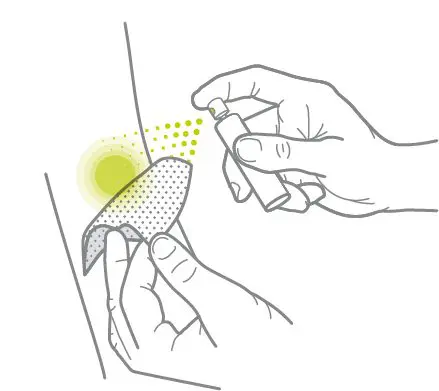
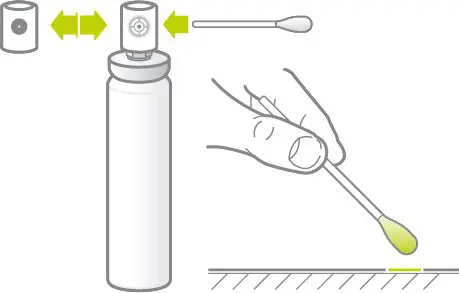
Wound Spray is suitable for the self-treatment of abrasions, cuts, burns, non-healing wounds and skin defects associated with different types of skin disorders.
Wound Spray is a 100% natural, preservative-free product enabling a non-toxic, painless non-touch application.