CHARLOTTE, N.C., and Arlington, VA – July 6, 2022 – Atrium Health’s Sanger Heart & Vascular Institute has received a Center of Excellence designation from Kerecis®, the company pioneering the use of fish skin and fatty acids in cellular therapy, tissue regeneration and protection. Sanger Heart & Vascular Institute surgeon Dr. Hector Crespo Soto is the first physician in the world to use SurgiBind implantable reinforcement fish-skin commercially. With this designation, Crespo Soto and the team at Sanger Heart & Vascular Institute can continue to lay the groundwork for centers around the country to utilize innovative technology to improve patient care and quality of life.

“The Center of Excellence designation and being the first in the world to use this type of graft technology in reconstructive surgery allows us to do more research and have more technology available to continue to use this technique to help patients,” said Crespo Soto.
Kerecis received authorization from the FDA to market Kerecis Omega3 SurgiBind™ in October 2021. Before the product was approved for civilian use, the technology had been used in the military to help heal wounded soldiers on the battlefield. Crespo Soto was involved in a pilot study in 2019 at Atrium Health Pineville to evaluate the new technology. Results of the study found the product decreased the need for patients to be admitted or undergo a skin graft operation, decreased the surface area of the wound, reduced pain and shortened healing and recovery time.
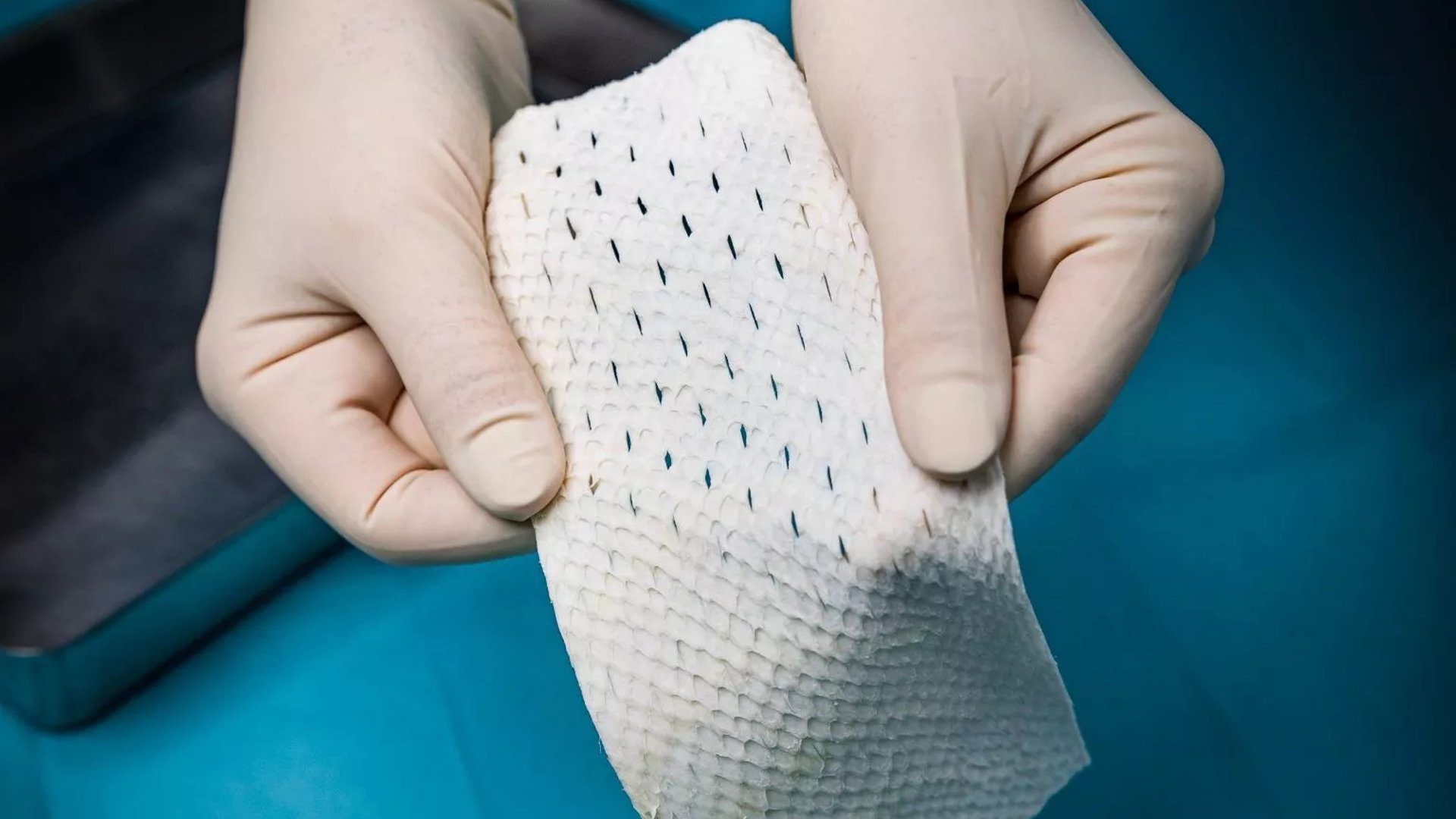
This technology, which is the first FDA-approved fish-skin implantable, uses the skin of north Atlantic cod. When grafted onto damaged human tissue, the fish skin recruits the body’s own cells and ultimately is converted into living tissue. The product is intended for implantation to reinforce soft tissue where weakness exists, such as in patients requiring soft tissue repair or reinforcement in plastic or reconstructive surgery.
Crespo Soto said this innovative technique creates an environment for the patient’s own tissue to regenerate. “The material resembles human skin – but it’s robust, pliable and tear-resistant. It can be easily cut or meshed for application and only requires brief hydration with saline before use,” said Crespo Soto.
The fish-skin device is Kerecis’s first implantable medical device created for the surgical market to help physicians better manage the risk of complications and improve results. The company has several fish skin products on the market that all are indicated for topical use. SurgiBind is the company’s first implantable product.
“We have seen very good clinical outcomes at Atrium Health’s Sanger Heart & Vascular Institute,” said Fertram Sigurjonsson, founder and CEO of Kerecis. “The partnership that we have with Sanger Heart & Vascular Institute is based on the principles of innovation and surgical excellence, both of which are very important for us. With the designation of excellence, we want to award facilities that put compassion in the spotlight by aiming for improved surgical outcomes for all patients.”
While the product can be used in a variety of ways, it is notably used to prevent amputation where the wound is located. Soto said the technique has had a tremendous impact on patients who are involved with Sanger’s innovative limb salvage program, which was launched in 2012 to reduce unnecessary or preventable amputations and save patients’ limbs.
Crespo Soto said since the study began in 2019, the fish-skin technology has been used in 200-300 cases a year where it has been used to treat patients and help drive positive outcomes.
“The team at Atrium Health’s Sanger Heart & Vascular Institute has been instrumental in making a great effort to take care of our patients,” said Crespo Soto. “I think this helps us expedite the care our patients need and helps us give our patients a better quality of life by allowing wounds to heal faster than they would with standard care.”
For more information about heart care at Atrium Health & Sanger Heart & Vascular Institute, please visit AtriumHealth.org/Heart
Atrium Health is a nationally recognized leader in shaping health outcomes through innovative research, education and compassionate patient care. Based in Charlotte, North Carolina, Atrium Health is an integrated, nonprofit health system with more than 70,000 teammates serving patients at 40 hospitals and more than 1,400 care locations. It provides care under the Atrium Health Wake Forest Baptist name in the Winston-Salem, North Carolina, region, as well as Atrium Health Navicent and Atrium Health Floyd in Georgia and Alabama. Atrium Health is renowned for its top-ranked pediatric, cancer and heart care, as well as organ transplants, burn treatments and specialized musculoskeletal programs. A recognized leader in experiential medical education and groundbreaking research, Wake Forest University School of Medicine is the academic core of the enterprise, including Wake Forest Innovations, which is advancing new medical technologies and biomedical discoveries. Atrium Health is also a leading-edge innovator in virtual care and mobile medicine, providing care close to home and in the home. Ranked nationally among U.S. News & World Report’s Best Hospitals in eight pediatric specialties and for rehabilitation, Atrium Health has also received the American Hospital Association’s Quest for Quality Prize and its 2021 Carolyn Boone Lewis Equity of Care Award, as well as the 2020 Centers for Medicare & Medicaid Services Health Equity Award for its efforts to reduce racial and ethnic disparities in care. With a commitment to every community it serves, Atrium Health seeks to improve health, elevate hope and advance healing – for all, providing $2.46 billion last year in free and uncompensated care and other community benefits.
Kerecis develops products from fish skin and fatty acids that protect and regenerate human wounds and heal damaged tissue. Because there is no risk of a viral-disease transfer from Atlantic cod to humans, the fish skin needs only mild processing for medical use and maintains its natural structure and elements.
A progressive and innovative company, Kerecis is committed to the United Nations Sustainable Development Goals. The fish skin used in Kerecis products derives from wild and sustainable fish stock caught in pristine Icelandic waters and processed with 100% renewable energy in the town of Isafjordur, close to the Arctic Circle.