Fish Skin for Burn Healing
Faster healing, potentially decreasing length of stay
GraftGuide, the cutting-edge solution from Kerecis, redefines burn care, offering unprecedented advantages for both patients and surgeons.
Kerecis GraftGuide® Products
Kerecis GraftGuide is intact fish skin especially developed for the management of burn wounds and donor sites.
The Kerecis fish-skin grafts contain fat, protein, elastin, glycans and other natural skin elements and are supplied in multiple shapes, variants and sizes.
The product classifies as a medical device and consist of a full thickness fish-skin that has been processed using Kerecis’ proprietary EnviroIntact™ method. The product is not subject to the May 31, 2021, FDA 351 regulation.

GraftGuide® Mano
The intact fish-skin graft in the shape of the hand to easily allow application on its complex 3D structure. Available in two sizes, medium and large, and in either left or right versions, for use in palmar or dorsum applications. Designed to reduce operation time when managing hand burn injuries.
GraftGuide Meshed 2:1
2:1 pre-meshed intact fish-skin graft designed to expand and cover larger wounds. Provides a viable clinical and economic solution for large wounds.
Contact Your Dedicated Sales Professional
Our Vision
To extend life by supporting the body’s own ability to regenerate.

Largest RCT on DFUs with Exposed Bone or Tendon Brings New Hope for Patients
Benefits of Using GraftGuide Products
Here’s why GraftGuide is indispensable for burn treatment:
Accelerated Recovery: GraftGuide streamlines graft application in burn cases, expediting healing and reducing patient recovery times. By facilitating precise placement, it optimizes the integration process, enabling patients to heal faster and return to their lives sooner.
Decreased Length of Stay: With GraftGuide, hospitals can significantly reduce the length of stay for burn patients. Its efficiency in graft application minimizes the need for prolonged hospitalization, leading to cost savings and improved resource allocation.
“GraftGuide’s efficiency in graft application minimizes the need for prolonged hospitalization…”
Coverage for Large Areas: GraftGuide is uniquely equipped to handle extensive burn injuries. Its adaptable design enables surgeons to cover large areas efficiently, providing comprehensive wound coverage with precision and ease.
Enhanced Patient Comfort: GraftGuide prioritizes patient comfort throughout the healing process.
See how Kerecis Helped Burn Survivor Pétur Oddsson
![]()
For these huge Necrotizing Fasciitis wounds, or huge degloving injuries from trauma, I’m using those larger [Kerecis meshed] pieces and we’re actually saving a lot of money by using those larger pieces.
– Dr. Erika Mabes, DO
Need more information?
From the town of Isafjordur in northwest Iceland, Kerecis develops, manufactures, and sells patented fish skin soft tissue regeneration products that have regulatory approval in the United States, Europe and several other jurisdictions.
Other Kerecis Products for Operating Room Use
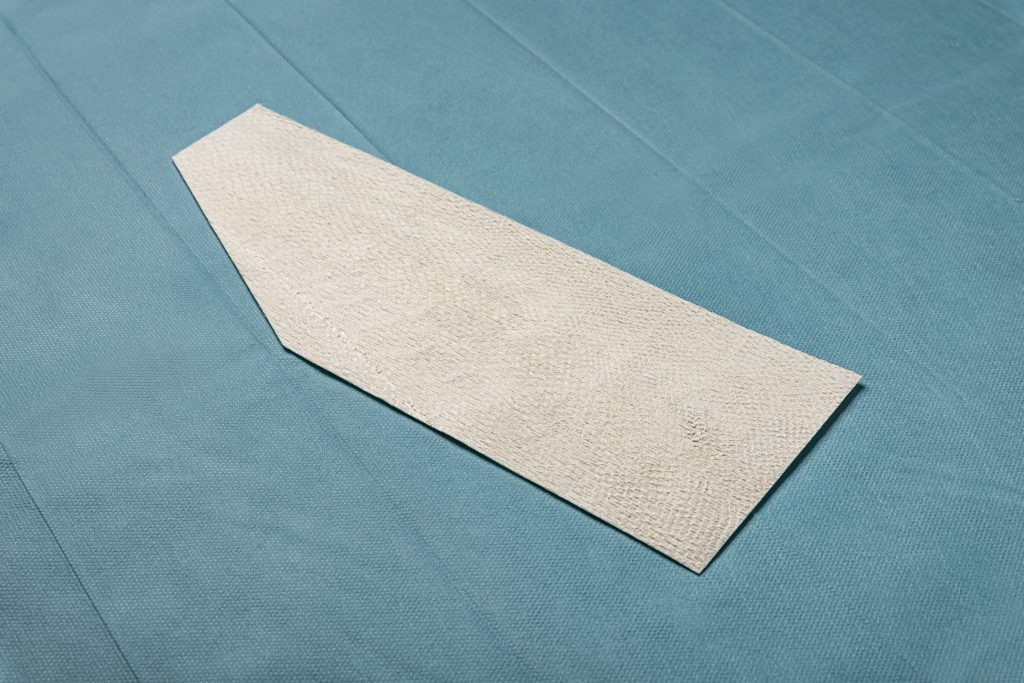
SurgiClose®
- Intact fish skin graft used for supporting tissue
regeneration on surgical, traumatic and acute wounds. - Easily adheres to complex wound beds and helps provide a natural
microbial barrier.2,3,6

SurgiClose®
Micro
- Intact Fish Skin that has been fragmented.
- Offers more surface area that non-fragmented grafts and is designed to adhere to and fill deep wound spaces and irregular geometries
- Demonstrates excellent ease of use and application characteristics, while also minimizing potential product waste.
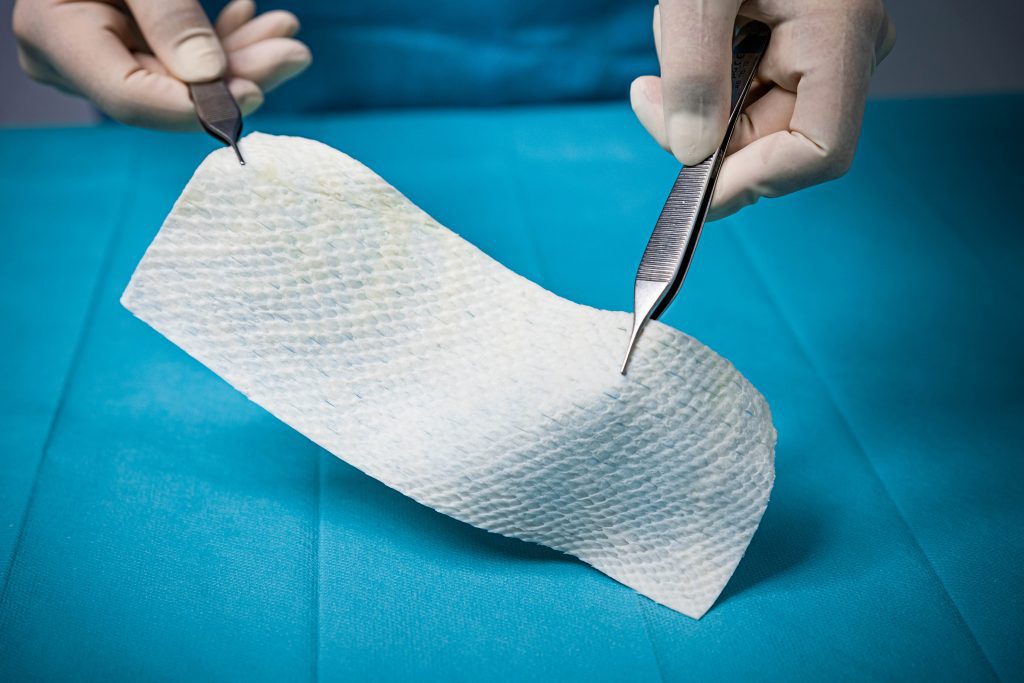
SurgiBind®
- Intact and implantable fish skin graft for tissue reinforcement and is FDA-approved as a medical device for surgical implantation.
- Aids in the reinforcement of soft tissue where weakness exists in plastic and reconstructive surgery.
User Information
Indication
• Partial and full thickness wounds.
• Trauma wounds, including abrasions, lacerations, second-degree burns, skin tears.
• Surgical wounds, including donor sites, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence.
The product derives from a from a fish source and should not be used in patients with a known allergy to fish or sensitivity. Note that fish-allergy is not the same as shellfish-allergy. The Shield product also contains silicone and should not be used for patient with silicone sensitivity.
The product and its variations are not available in all countries and may be known under different trade names. Products, product names, indications and claims vary between jurisdictions. Contact your Kerecis representative if you have questions about the availability of Kerecis products in your area.
Always make sure to check with your local health care professional and read the actual printed package information prior to use. Information on this website might not be current and might not be an accurate reflection of current product labeling.
This website is not intended to provide diagnosis, treatment or medical advice. Products, services, information and other content provided on this website, including information that may be provided on directly or by linking to third-party websites, are provided for informational purposes only. Information on this website should not be considered as a substitute for advice from a healthcare professional.
Consult with a physician or other healthcare professional regarding any medical or health related diagnosis or treatment options.
A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Kerecis does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before using it in surgery.
The information presented on this website are intended to demonstrate the Kerecis product offerings. A surgeon must always refer to the package insert, product label and/or instructions for use before using any Kerecis product.
